基于碱(土)金属化合物的抑制剂对氢化铝爆炸的抑制机理和效果评估
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
不稳定的氢演化和爆炸风险限制了 AlH3 的安全生产和广泛应用。碱(土)金属化合物(A(E)MCs)具有储量丰富、毒性低和环境友好等特点,是抑制 AlH3 爆炸的理想材料。目前,还缺乏对抑爆效果(EIE)的系统评价和对抑爆机理的了解。本研究利用 A(E)MCs的热性能来选择抑制剂,并分析了不同基团对 AlH3 的 EIE,从而研究了 16 种 A(E)MCs 对 AlH3 爆炸的抑制行为。值得注意的是,KH2PO4 能有效地将爆炸强度降低到 0.53 MPa-m/s,最大压力和最大压力上升率分别为 0.68 MPa 和 4.63 MPa/s。EIE 可通过颗粒 Al2O3 的相对变化来定量描述,阻止颗粒 Al2O3 的形成可有效改善 EIE。结合表征和模拟结果可以发现,A(E)MCs 可从化学和物理两方面抑制 AlH3 爆炸。此外,合成的复合抑制剂 KH2PO4/SiO2 可吸附火焰自由基,使爆炸强度降低 92.23%,EIE 提高 4.33%。希望我们的工作能为储氢材料的安全应用和 EIE 的定量评估提供理论支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
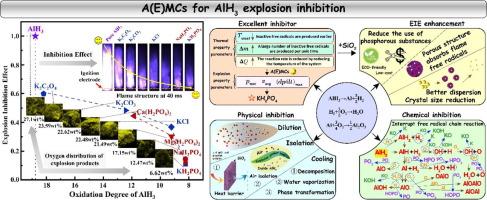
Inhibition mechanism and effect assessment of alkali (Earth) metal compound-based inhibitors on aluminum hydride explosion
Safe production and widespread application of AlH3 are limited by unstable hydrogen evolution and explosion risk. Alkali (earth) metal compounds, A(E)MCs, are promising materials for inhibiting AlH3 explosions due to abundant reserves, low toxicity, and environmentally friendly. Currently, systematic evaluation of explosion inhibition effect (EIE) and understanding of inhibition mechanism is lacking. This study examines the inhibition behavior of 16 A(E)MCs for AlH3 explosions using thermal properties of A(E)MCs to select inhibitors, and EIE of different groups on AlH3 is analyzed. Notably, KH2PO4 effectively reduces explosion intensity to 0.53 MPa·m/s, with the maximum pressure and maximum pressure rise rate of 0.68 MPa and 4.63 MPa/s. EIE can be quantitatively described by relative changes in particle Al2O3, and preventing the formation of particle Al2O3 can be effective in improving EIE. Combining characterizations and simulation results reveals that A(E)MCs inhibit AlH3 explosions in both chemical and physical ways. Further, the synthesized composite inhibitor KH2PO4/SiO2, which adsorbs flame radicals, reduces explosion intensity by 92.23 % and enhances EIE by 4.33 %. We hope our work can provide theoretical support for the safe application of hydrogen storage materials and the quantitative assessment of EIE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


