大叶女贞的生物碱和一种新的不饱和羧酸
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
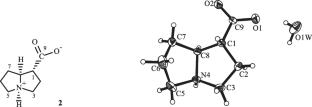
首次从Lindelofia macrostyla (Bunge) Popov中分离出了新的生物碱lindelofamine N-氧化物(1)和已知的1-外-羧基吡咯烷(2)以及异喹啉生物碱O-methylarmepavine methyliodide的季盐(3)。通过核磁共振光谱确定了它们的结构,并利用 X 射线衍射分析确定了最后两个手性中心的绝对构型,分别为 1R、4R、8S 和 1S。此外,还分离出了新的羧酸 (2S,3R)-2-羟基-2-异丙基-3-{[(E)-2-甲基丁-2-烯酰]氧基}丁酸,其结构和绝对构型 (2S,3R) 已通过核磁共振光谱和 XSA 得到证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Alkaloids of Lindelofia macrostyla and a New Unsaturated Carboxylic Acid
The new alkaloid lindelofamine N-oxide (1) and the known 1-exo-carboxypyrrolizidine (2) and the quaternary salt of the isoquinoline alkaloid O-methylarmepavine methyliodide (3) were isolated from Lindelofia macrostyla (Bunge) Popov for the first time. The structures were established by NMR spectroscopy, the absolute configuration was determined using X-ray diffraction analysis of the chiral centers of the last two as 1R,4R,8S and 1S, respectively. The new carboxylic acid (2S,3R)-2-hydroxy-2-isopropyl-3-{[(E)-2-methylbut-2-enoyl]oxy}butanoic acid, the structure and absolute configuration (2S,3R) of which were proven by NMR spectroscopy and an XSA, was also isolated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


