氮杂环甘草醇的酰化作用
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
氮杂环庚-11-脱氧甘草亭醇的酰化反应合成了 N-乙酰基和 N,O-双乙酰基衍生物。氮杂环庚烷-11-脱氧甘油三醇的结构由 XSA 证实。有关其对细胞周期进展影响的数据表明,抑制细胞存活主要是由于细胞抑制作用,这种作用与 S 期或 G1 期的停滞有关,具体取决于细胞系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
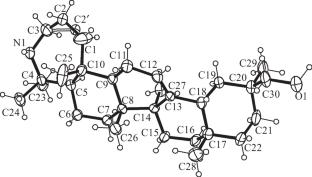
Acylation of Azepanoglycyrrhetol
Acylation of azepano-11-deoxoglycyrrhetol synthesized N-acetyl- and N,O-bis-acetyl derivatives. The structure of azepano-11-deoxoglycyrrhetol was confirmed by an XSA. Data for its influence on cell cycle progression suggested that suppression of cell survival was due primarily to a cytostatic effect that was related to arrest of the S or G1 phase, depending on the cell line.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


