在铜促进的 Ni/Al2O3-ZrO2 催化剂上进行乙醇干转化以生产富氢合成气
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
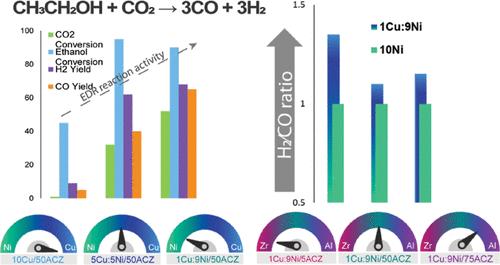
在催化乙醇干重整(EDR)工艺中,开发具有高抗烧结和抗碳沉积能力的稳定镍基催化剂是一项挑战。一种有效而实用的策略是引入第二种金属以获得镍基双金属催化剂。在本研究中,成功开发了以 Al-Zr-Ce(ACZ)复合氧化物为载体的铜镍双金属纳米颗粒,作为通过 EDR 生产合成气的多功能催化剂,并与以 ACZ 氧化物为载体的单金属镍和铜催化剂进行了比较。在催化剂中添加少量铜(1%)可形成结晶尺寸在 10 纳米到 30 纳米之间的铜镍合金,表现出较高的金属-支撑相互作用和抗烧结性。然而,由于乙醇分解的副反应导致催化剂失活,高铜含量限制了催化剂的活性。在 H2/CO = 1.1 时,催化剂 1Cu-9Ni/50ACZ 的 H2 和 CO 产率最高(T = 750 °C 时分别为 78% 和 70%)。铜的加入通过改变水气变换(WGS)反应途径和增加镍的还原性和分散性提高了 H2/CO 比率,这归因于铜镍合金的形成。Cu-Ni 合金在 WGS 反应中十分活跃,在乙醇的脱水和脱氢过程中与 Ni 具有协同作用,从而影响了产物的分布。此外,铜还在还原镍的碳化物形式(石墨化焦炭的前体)中发挥作用。研究还发现,载体成分对双金属催化剂的活性和稳定性有显著影响。研究表明,载体中的 Al/Zr 比率可以调整活性相的结晶尺寸,从而影响镍和铜的表面浓度及其比率,并决定了有助于气化所形成的焦炭的活性氧比率。这项工作为设计具有功能金属位点的高选择性催化剂提供了一种策略,这种催化剂可用于氢气或合成气的生产,同时调节 H2/CO 的比例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ethanol Dry Reforming for Hydrogen-Rich Syngas Production over Cu-Promoted Ni/Al2O3–ZrO2 Catalysts
Development of stable Ni-based catalysts with high resistance to sintering and carbon deposition is a challenge in the catalytic ethanol dry reforming (EDR) process. An effective and practical strategy is to introduce a second metal to obtain Ni-based bimetallic catalysts. In this study, bimetallic Cu–Ni nanoparticles supported on Al–Zr–Ce (ACZ) complex oxides were successfully developed as a multifunctional catalyst for syngas production via EDR and were compared with monometallic Ni and Cu catalysts supported on ACZ oxides. The addition of a small amount of copper (1%) to the catalyst resulted in the formation of a Cu–Ni alloy with crystallite sizes ranging from 10 to 30 nm, exhibiting a high metal–support interaction and resistance to sintering. However, a high Cu content limited the activity of the catalysts due to side reactions of ethanol decomposition, which led to catalyst deactivation. The catalyst 1Cu–9Ni/50ACZ exhibited the highest H2 and CO yields (78% and 70%, respectively, at T = 750 °C) at H2/CO = 1.1. The addition of Cu enhanced the H2/CO ratio by shifting the water–gas shift (WGS) reaction pathway and increasing the reducibility and dispersibility of Ni, which is attributed to the formation of a Cu–Ni alloy. The Cu–Ni alloy is active in the WGS reaction and has a synergistic effect with Ni in dehydration and dehydrogenation of ethanol, which affects the product distribution. Furthermore, copper plays a role in the reduction of carbide forms of nickel, which are precursors of graphitized coke. The support composition was also found to have a significant effect on the activity and stability of the bimetallic catalysts. It was demonstrated that the Al/Zr ratio in the support enables tuning the crystallite size of the active phase, which affects the surface concentrations of nickel and copper and their ratio and determines the ratio of reactive oxygen species that contribute to the gasification of the formed coke. This work provides a strategy to design highly selective catalysts with functional metal sites for hydrogen or syngas production with a regulated H2/CO ratio.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


