CD39 和 CD73 的拮抗剂可增强强力霉素的重新定位作用,从而诱导有效的抗肿瘤免疫反应。
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
研究报告指出,肿瘤免疫微环境(TiME)的细胞代谢是一个关键的检查点,干扰/支持抗癌免疫。细胞外 ATP(eATP)可介导抗癌免疫反应;然而,它在外切核苷酸酶的分解作用下会产生具有免疫抑制作用的腺苷。在本研究中,我们尝试在使用或不使用外切核苷酸酶拮抗剂的情况下重新使用强力霉素,以减轻 ATP 代谢和免疫抑制。这种方法对 eATP 和腺苷水平进行了量化。骨髓衍生的 M1 和 M2 极化巨噬细胞被保存在肿瘤模拟条件(TMC)中。总/CD4+T细胞与巨噬细胞共同培养,以了解强力霉素和/或外核苷酸酶拮抗剂对T细胞/亚群分化的影响。在 4 例 T1 诱导的乳腺癌中,对多西环素和/或外核苷酸酶拮抗剂及其协同作用的临床前疗效进行了评估。我们发现,多西环素通过降低 CD206+M2 巨噬细胞的频率来操纵巨噬细胞的极化,从而增强了 CD4+ 定向 CD8+ T 细胞的反应。多西环素减轻了CD39和CD73的表达,挽救了ATP分解。强力霉素通过增强 F4/80+ CD86+ M1 巨噬细胞和随后的抗肿瘤 Tbet+ CD4+T 细胞,减弱 FOXP3+ 调节性 T 细胞的频率,从而延缓肿瘤的生长,ARL67156 和 AMPCP(CD39 和 CD73 拮抗剂)对此起到了协同作用。观察到 ARL67156 和 AMPCP 的协同作用,表明有可能单独使用强力霉素或与外切核苷酸酶拮抗剂联合使用,以实现腺苷介导的免疫抑制。随后,我们的研究结果表明,多西环素是一种新型代谢检查点阻断剂(IMB),可用于对抗外切核苷酸酶,并可作为一种单药疗法或与外切核苷酸酶拮抗剂联合使用作为一种 IMB 进行适当的改良/给药。本文章由计算机程序翻译,如有差异,请以英文原文为准。
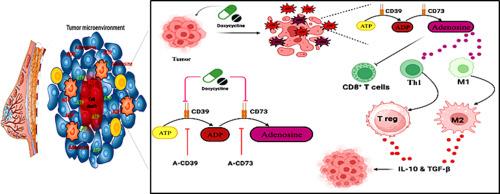
Antagonists of CD39 and CD73 potentiate doxycycline repositioning to induce a potent antitumor immune response
Studies have reported that cellular metabolism at the tumor-immune microenvironment (TiME) serves as a critical checkpoint and perturbs/supports anti-cancer immunity. Extra cellular ATP (eATP) may mediate anti-cancer immune response; however, its catabolism by ectonucleotidase generates immunosuppressive adenosine. In the presented work, we have tried to repurpose doxycycline with or without an antagonist of ectonucleotidase for mitigating ATP metabolism and immunosuppression. In this methodology eATP and adenosine levels were quantified. Bone marrow-derived M1 and M2 polarized macrophages were maintained in tumor mimicking condition (TMC). Total/CD4+Tcells were co-cultured with macrophages to understand the impact of doxycycline and/or antagonist of ectonucleotidase on T cell/subset differentiation. Preclinical efficacy of doxycycline and/or ectonucleotidase antagonist and their synergy was scored in 4T1-induced breast carcinoma. We found that Doxycycline manipulated macrophage polarization by decreasing the frequency of CD206+M2 macrophages, which resulted in enhanced CD4+ directed CD8+ T cell response. Doxycycline alleviated the expression of CD39 and CD73, rescuing ATP catabolism. Doxycycline delayed tumor growth by enhancing F4/80+ CD86+ M1 macrophages and subsequently anti-tumor Tbet+ CD4+Tcells, attenuating the frequency of FOXP3+ regulatory T cells, which was cooperatively supported by ARL67156 and AMPCP (CD39 and CD73 antagonist).A synergy was observed with ARL67156 and AMPC Pensuring a possibility of using doxycycline alone or in combination with an antagonist of ectonucleotidase to present adenosine-mediated immunosuppression. Subsequently, our finding indicated that prospective usage of doxycycline as a novel metabolic checkpoint blocker (IMB) against ectonucleotidase and may be modified/delivered appropriately as a monotherapy or in combination with antagonists of ectonucleotidases as an IMB.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


