利用荧光素衍生物对 Pb2+ 进行比色和荧光检测。
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-11-01
DOI:10.1016/j.saa.2024.125373
引用次数: 0
摘要
本研究使用一种简单的荧光探针--生物素-4-荧光素,开发了一种简单、低成本的快速检测 Pb2+ 的方法。Pb2+ 的存在会导致荧光素的颜色从黄色变为粉红色,这可以通过肉眼直观地检测到。在与其他金属离子(如 Fe3+、Cu2+、Ca2+、Co2+ 和 Cd2+)一起测试时,未观察到生物素-4-荧光素的颜色变化,这表明荧光素衍生物对 Pb2+ 具有选择性。此外,pH 值高于 7.0 时颜色会发生变化,pH 值升高时粉红色更浓。在生物素-4-荧光素/Pb2+溶液中加入螯合试剂 EDTA 会使颜色恢复到游离生物素-4-荧光素的原色。荧光素的荧光光谱随 Pb2+浓度的增加而减弱,pH 值越高淬灭越强。根据荧光光谱计算出的关联常数(Ka)为 2.00 × 104 M-1,检出限(LOD)和定量限(LOQ)分别为 1.38 × 10-5 M(2.86 ppm)和 4.61 × 10-5 M(9.55 ppm)。约伯图分析表明结合相互作用的比例为 2:1。用这种方法对未经处理的废水中的 Pb2+ 进行了定量分析,结果为 24.99 ppm。这种快速分析有利于在实际样品溶液(如工业工厂废水)中进行铅检测。本文章由计算机程序翻译,如有差异,请以英文原文为准。
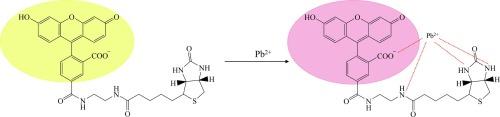
Colorimetric and fluorescence detection of Pb2+ by fluorescein derivative
A simple and low cost method for rapid detection of Pb2+ was developed in this study using a simple fluorescent probe, biotin-4-fluorescein. The presence of Pb2+ induced the color change of fluorescein from yellow to pink which can be detected visually by naked eyes. The color change of biotin-4-fluorescein was not observed while testing with other metal ions, such as Fe3+, Cu2+, Ca2+, Co2+ and Cd2+, indicating the selectivity of fluorescein derivative with Pb2+. Moreover, the color change was observed at pH above 7.0, and the pink color got more intense when increasing pH. Adding EDTA, a chelating reagent, into the solution of biotin-4-fluorescein/Pb2+ resulted to the rebound of the color to the original color of free biotin-4-fluorescein. The fluorescence spectra of fluorescein decreased with increasing Pb2+concentration, and the quenching was enhanced at higher pH. The association constant (Ka) of 2.00 × 104 M−1 was calculated from the fluorescence spectra with the limit of detection (LOD) and the limit of quantitation (LOQ) of 1.38 × 10−5 M (2.86 ppm) and 4.61 × 10−5 M (9.55 ppm), respectively. Job’s plot analysis indicated 2:1 ratio of binding interaction. Pb2+ in untreated wastewater was quantified for 24.99 ppm using this method. This quick analysis can be beneficial for the application of lead detection in real sample solutions, such as wastewater from industrial factories.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


