BioID 接近性图谱揭示了前接合体复合体中新型 SAP18 相互作用。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-11-01
DOI:10.1016/j.bbrc.2024.150944
引用次数: 0
摘要
SAP18 蛋白最初是与 SIN3 转录抑制复合体结合在一起被发现的。随后的生化分馏研究发现,SAP18 是另一个不同的三聚体复合物(称为凋亡和剪接相关蛋白(ASAP)复合物)的组成部分。SAP18在不同复合物中的存在突显了它在转录和剪接调控中的双重作用。在我们的研究中,我们旨在利用近距离依赖性生物素鉴定(BioID)来定义 SAP18 的体内相互作用组。对链霉亲和素纯化的生物素化蛋白质进行的质谱分析发现了新的与 SIN3 相关的相互作用者,包括 RBBP4 和 SAP30BP。值得注意的是,我们发现有 72 个剪接体蛋白与 SIN3 高度富集。此外,互补免疫沉淀试验验证了 SAP18 与剪接体前成分 SNRNP70、SNRPA、SF3B1、U2AF1 和 SR 蛋白 SRSF1 的新型相互作用。使用已知缺乏 ASAP 相互作用的 C 端 SAP18 双点突变体进行的突变分析表明,SAP18 与核苷酸前体蛋白的相互作用减弱。总之,我们的研究结果使人们对 SAP18 的相互作用组有了更深入的了解,揭示了它在与 ASAP 成分结合时与预拼接体的关联。本文章由计算机程序翻译,如有差异,请以英文原文为准。
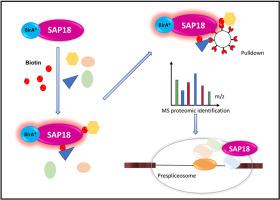
BioID proximity mapping reveals novel SAP18 interactions in the prespliceosomal complex
SAP18 protein was originally discovered in association with the SIN3 transcriptional repressor complex. Subsequent biochemical fractionation studies identified SAP18 as a component of another distinct trimeric complex termed as the apoptosis- and splicing-associated protein (ASAP) complex. The existence of SAP18 in distinct complexes highlights its dual role in transcriptional and splicing regulation. In our study, we aim to define the in vivo interactome of SAP18 using proximity-dependent biotin identification (BioID). Mass spectrometry analysis of streptavidin-purified biotinylated proteins revealed new SIN3-associated interactors, including RBBP4 and SAP30BP. Notably, we identified 72 spliceosomal proteins as highly enriched interactors. Additionally, a complementary immunoprecipitation assay validated novel interactions of SAP18 with the prespliceosomal components SNRNP70, SNRPA, SF3B1, U2AF1, and the SR protein SRSF1. Mutational analysis using a C-terminal SAP18 double point mutant, which is known to be deficient in ASAP-interaction, demonstrated a debilitated interaction with the prespliceosomal proteins. Altogether, our results present a refined understanding of the SAP18 interactome, uncovering its association with the prespliceosome in conjugation with ASAP components.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


