缬氨霉素基钾选择膜与氯化钾水溶液界面的电荷转移电阻
IF 8
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
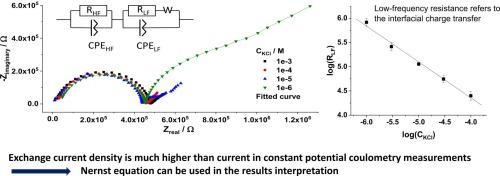
电化学阻抗能谱(EIS)研究了含有中性离子源缬氨霉素和作为离子交换剂的四(对氯苯基)硼酸钾膜的钾选择性电极(K-ISE)。当 ISE 与浓度为 10-4 M 或更高的氯化钾溶液接触时,在相应的奈奎斯特图中只出现一个高频半圆。当 ISE 与浓度低于 10-4 M 的氯化钾溶液接触时,得到的奈奎斯特图中也有清晰的低频半圆。RLF 值(低频电阻)与溶液中 KCl 浓度之间的规律性依赖关系允许将 RLF 归因于膜/溶液界面上的电荷转移电阻。在非零电流模式下,根据 RLF 值估算出的交换电流密度明显大于分析应用中流经 ISE 的小电流。这表明,电流流动并没有破坏界面电化学平衡,这有利于使用内斯特方程来解释恒电位库仑测量法等获得的数据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Charge transfer resistance at the interface between valinomycin-based potassium-selective membranes and KCl aqueous solutions
Potassium-selective electrodes (K-ISEs) with membranes containing neutral ionophore valinomycin and potassium tetrakis(p-chlorophenyl)borate as ion-exchanger, in different concentrations, were studied by the electrochemical impedance spectroscopy (EIS). When ISEs are in contact with KCl solutions with concentrations of 10−4 M and higher, only a high-frequency semicircle is present in the respective Nyquist plots. Nyquist plots obtained with ISEs in contact with KCl solutions with concentrations below 10−4 M contain also well-resolved low-frequency semicircles. A regular dependence of the values of RLF - the low-frequency resistance on the concentration of KCl in solution allowed ascribing RLF to the charge transfer resistance at the membrane/solution interface. The exchange current densities estimated from the RLF values are significantly larger than small currents flowing through ISEs in their analytical applications under non-zero current modes. This suggests that the interfacial electrochemical equilibrium is not violated by the current flow, in favor of using the Nernst equation for the interpretation of the data obtained by, e.g., the constant potential coulometry method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


