OSU-T315通过靶向ILK/NF-κB信号通路提高免疫治疗效果,从而克服三阴性乳腺癌的免疫抑制。
IF 4.8
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
三阴性乳腺癌(TNBC)是一种具有侵袭性和免疫原性的乳腺癌亚型。由于缺乏生物标志物,免疫检查点抑制剂(ICIs)在这种类型的乳腺癌中具有广阔的应用前景。肿瘤微环境(TME)中表达转录因子叉头盒P3(Foxp3)的调节性T细胞(Tregs)的浸润是导致ICIs耐药的关键因素。因此,消除肿瘤抗原特异性Tregs可能是提高ICIs疗效的一个重要方面。本研究基于基因表达总库(Gene Expression Omnibus)和癌症基因组图谱(The Cancer Genome Atlas)数据库,结合体内和体外实验模型,验证了整合素连接激酶(ILK)在TNBC中的高表达是导致Foxp3+-Tregs在TME中高浸润的关键差异因素。随后,我们选择了ILK特异性抑制剂OSU-T315进行体外和体内干预。重要的是,我们发现OSU-T315通过抑制ILK/NF-κB通路阻断了肿瘤细胞中CCL17/CCL22的分泌,从而导致Foxp3+-Tregs的凋亡和程序性细胞死亡-1(PD-1)表达的减少。因此,我们的研究结果表明了OSU-T315的一种新机制,有望应用于TNBC的治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
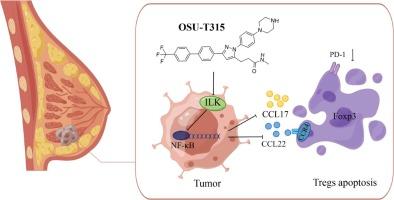
OSU-T315 overcomes immunosuppression in triple-negative breast cancer by targeting the ILK/NF-κB signaling pathway to enhance immunotherapeutic efficacy
Triple negative breast cancer (TNBC) is an aggressive and immunogenic subtype of breast cancer. The absence of biomarker has given immune checkpoint inhibitors (ICIs) a broad prospect in this type of breast cancer. The infiltration of regulatory T cells (Tregs) expressing transcription factor forkhead box P3 (Foxp3) in the tumor microenvironment (TME) is the key factor leading to ICIs resistance. Therefore, elimination of tumor antigen-specific Tregs may be an important aspect of improving ICIs efficacy. In this study, it based on the Gene Expression Omnibus and The Cancer Genome Atlas database, along with in vivo and in vitro experimental models, to verified that the high expression of integrin-linked kinase (ILK) in TNBC is the key differential factor leading to the high infiltration of Foxp3+-Tregs in the TME. Then, we selected ILK-specific inhibitor, OSU-T315, to intervene in vitro and vivo. Importantly, we found that OSU-T315 blocked the secretion of CCL17/CCL22 in tumor cells by inhibiting the ILK/NF-κB pathway, resulting in the apoptosis of Foxp3+-Tregs and decreased programmed cell death-1 (PD-1) expression. Therefore, our findings indicate a novel mechanism of OSU-T315 with potential therapeutic application in TNBC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


