构建具有 Bi-N4 位点和 TCPP(Bi)@HKUST-1 衍生的协同 Bi 簇的铜铋催化剂,以提高甲醛制丁炔二醇的效果
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
构建能在还原气氛中有效催化的功能催化剂是一项相当具有挑战性的工作。本研究制备了铜和铋之间由 Bi-N4 桥接的催化剂。在这种催化剂中,Bi-N4 和铋团簇位点与铜纳米颗粒(BiSAC&Clu-Cu-NC)一起被纳入多孔碳氮网络。在合成过程中,采用了金属卟啉修饰的 HKUST-1 (TCPP(Bi)@HKUST-1)作为前体。此外,金属卟啉在催化剂制备过程中起到了封盖剂的作用,从而提高了煅烧后铋物种的分散性。值得注意的是,在乙炔和甲醛还原气氛中,BiSAC&Clu-Cu-NC 催化剂在 20 小时内对 1,4-丁炔二醇的选择性达到 93.8%,Bi 的负载量为 0.6 wt%。此外,还根据实验表征和 DFT 计算提出了一个机理模型,以阐明所观察到的协同催化行为。该模型被称为 "具有团簇和单金属位点的纳米颗粒"(NCS 机制)。在材料基质中保留 Bi 簇结构对增强甲醛的吸附和活化起着关键作用。不同铜种之间的界面效应有利于活化反应底物乙炔。此外,Bi-N4 结构还可以作为一个重要的通道,促进铜和 Bi 元素之间的电子转移,从而降低关键反应中间产物的活化能垒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
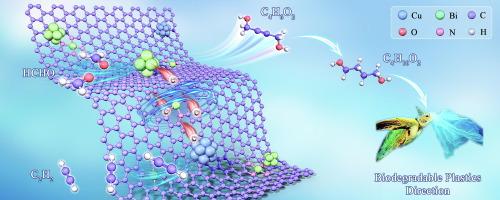
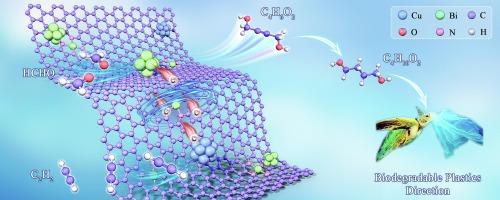
Construction of a copper-bismuth catalyst featuring Bi-N4 sites and synergistic Bi clusters derived from TCPP(Bi)@HKUST-1 for enhanced formaldehyde to butynediol
The construction of functional catalysts that efficiently catalyze in a reducing atmosphere is considered rather challenging. In this study, the catalysts bridged by Bi-N4 between copper and bismuth species were prepared. Herein, Bi-N4 and bismuth cluster sites were incorporated into porous carbon–nitrogen networks along with copper nanoparticles (BiSAC&Clu-Cu-NC). For the synthesis, metalloporphyrin-modified HKUST-1 (TCPP(Bi)@HKUST-1) was employed as a precursor. Additionally, metalloporphyrin functioned as a capping agent during catalyst preparation, thereby enhancing the dispersion of Bi species after calcination. Notably, the BiSAC&Clu-Cu-NC catalyst demonstrated 93.8 % selectivity towards 1,4-butynediol over 20 h at a Bi loading of 0.6 wt% in a reducing atmosphere of acetylene and formaldehyde. Furthermore, a mechanistic model is proposed that elucidates the observed synergistic catalytic behavior based on experimental characterization and DFT calculations. This proposed model is termed “Nanoparticles with Cluster and Single Metal Sites”(NCS mechanism). The retention of the Bi cluster structure within the material matrix plays a pivotal role in enhancing the adsorption and activation of formaldehyde. Interfacial effects between different copper species favour the activation of the reaction substrate acetylene. Additionally, the Bi-N4 structure can function as a crucial conduit, facilitating electron transfer between Cu and Bi elements and consequently lowering the activation energy barrier for key reaction intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


