I 类谷胱甘肽的二硫醇机制促进了谷胱甘肽作为还原剂的特异性。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
I 类谷胱甘肽在还原型谷胱甘肽(GSH)的帮助下,通过单硫醇机制或二硫醇机制可逆地还原谷胱甘肽和非谷胱甘肽二硫化物。单硫醇机制只涉及单个谷胱甘肽化的活性位点半胱氨酰残基,而二硫醇机制则需要在活性位点半胱氨酰残基和解析半胱氨酰残基之间额外形成分子内二硫键。虽然谷胱甘肽二硫化物底物对谷胱甘肽的氧化作用已经有了广泛的表征,但 S-谷胱甘肽化谷胱甘肽或分子内谷胱甘肽二硫化物还原过程中酶与底物之间的相互作用仍鲜为人知。在这里,我们比较了还原 S-谷胱甘肽化谷胱甘肽和分子内谷胱甘肽二硫化物的硫醇特异性。我们发现,S-谷胱甘肽化的谷拉糖苷酶能迅速与大量硫醇发生反应,而 I 类谷拉糖苷酶的第二个谷胱甘肽相互作用位点缺乏对 GSH 作为还原剂的特异性。与此相反,部分分子内紧张的谷胱甘肽二硫化物的缓慢还原涉及与第 1 个谷胱甘肽相互作用位点上的 GSH 的两个羧基的特异性相互作用。因此,I类谷胱甘肽的二硫醇机制促进了GSH作为还原剂的特异性,这或许可以解释原核生物和真核生物中普遍存在二硫醇谷胱甘肽的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
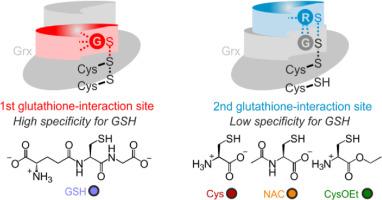
The dithiol mechanism of class I glutaredoxins promotes specificity for glutathione as a reducing agent
Class I glutaredoxins reversibly reduce glutathione- and nonglutathione disulfides with the help of reduced glutathione (GSH) using either a monothiol mechanism or a dithiol mechanism. The monothiol mechanism exclusively involves a single glutathionylated active-site cysteinyl residue, whereas the dithiol mechanism requires the additional formation of an intramolecular disulfide bond between the active-site cysteinyl residue and a resolving cysteinyl residue. While the oxidation of glutaredoxins by glutathione disulfide substrates has been extensively characterized, the enzyme-substrate interactions for the reduction of S-glutathionylated glutaredoxins or intramolecular glutaredoxin disulfides are still poorly characterized. Here we compared the thiol-specificity for the reduction of S-glutathionylated glutaredoxins and the intramolecular glutaredoxin disulfide. We show that S-glutathionylated glutaredoxins rapidly react with a plethora of thiols and that the 2nd glutathione-interaction site of class I glutaredoxins lacks specificity for GSH as a reducing agent. In contrast, the slower reduction of the partially strained intramolecular glutaredoxin disulfide involves specific interactions with both carboxylate groups of GSH at the 1st glutathione-interaction site. Thus, the dithiol mechanism of class I glutaredoxins promotes specificity for GSH as a reducing agent, which might explain the prevalence of dithiol glutaredoxins in pro- and eukaryotes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


