通过仿生纳米粒子靶向补充 NAD+ 可同时治疗内膜增生和加速内皮再形成:面向下一代内皮保护和抗血管再狭窄疗法的概念验证研究。
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
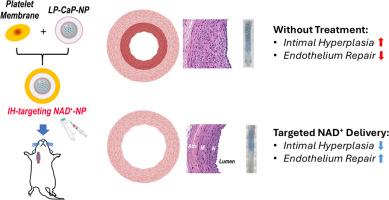
血管内介入治疗经常因再狭窄而失败,再狭窄主要是由平滑肌细胞(SMC)增殖导致内膜增生(IH)引起的。目前预防再狭窄的策略远非完美,而且会对脆弱的内皮细胞(EC)造成严重的附带损伤,导致严重的血栓风险。烟酰胺腺嘌呤二核苷酸(NAD+)是一种辅酶和信号底物,与氧化还原和代谢平衡有关,在预防心血管疾病方面具有多重作用。然而,NAD+ 的补充与 IH 和 EC 再生这微妙的二重奏之间的功能性联系尚未确立。由于 NAD+ 的细胞摄取能力差、生物利用率低,因此补充 NAD+ 一直是一项挑战。我们最近发明了第一种纳米载体,可在体内直接在细胞内输送 NAD+。结合这种原型 NAD + 负载磷酸钙(CaP)纳米粒子(NP)和仿生表面功能化的优点,我们创造了一种具有血小板膜涂层的仿生 P-NAD + -NP,从而实现了一种针对 IH 的注射方式,并具有良好的生物相容性。利用人体细胞原代培养,我们证明了 NP 辅助 NAD+ 补充在选择性抑制主动脉 SMC 过度增殖方面的益处,同时还能在不同程度上保护主动脉 EC 免受凋亡。此外,在大鼠球囊血管成形术模型中,与对照组(即生理盐水、游离 NAD+ 溶液、空 CaP-NP)相比,血管成形术后立即静脉注射 P-NAD + -NP 的单剂量治疗不仅减轻了 IH,还加速了体内 EC 的再生(再内皮化)。总之,我们目前的研究提供了概念证明,支持靶向 NAD+ 补充纳米疗法在控制再狭窄和改善再内皮化方面的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeted NAD+ repletion via biomimetic nanoparticle enables simultaneous management of intimal hyperplasia and accelerated re-endothelialization: A proof-of-concept study toward next-generation of endothelium-protective, anti-restenotic therapy
Endovascular interventions often fail due to restenosis, primarily caused by smooth muscle cell (SMC) proliferation, leading to intimal hyperplasia (IH). Current strategies to prevent restenosis are far from perfect and impose significant collateral damage on the fragile endothelial cell (EC), causing profound thrombotic risks. Nicotinamide adenine dinucleotide (NAD+) is a co-enzyme and signaling substrate implicated in redox and metabolic homeostasis, with a pleiotropic role in protecting against cardiovascular diseases. However, a functional link between NAD+ repletion and the delicate duo of IH and EC regeneration has yet to be established. NAD+ repletion has been historically challenging due to its poor cellular uptake and low bioavailability. We have recently invented the first nanocarrier that enables direct intracellular delivery of NAD+ in vivo. Combining the merits of this prototypic NAD+-loaded calcium phosphate (CaP) nanoparticle (NP) and biomimetic surface functionalization, we created a biomimetic P-NAD+-NP with platelet membrane coating, which enabled an injectable modality that targets IH with excellent biocompatibility. Using human cell primary culture, we demonstrated the benefits of NP-assisted NAD+ repletion in selectively inhibiting the excessive proliferation of aortic SMC, while differentially protecting aortic EC from apoptosis. Moreover, in a rat balloon angioplasty model, a single-dose treatment with intravenously injected P-NAD+-NP immediately post angioplasty not only mitigated IH, but also accelerated the regeneration of EC (re-endothelialization) in vivo in comparison to control groups (i.e., saline, free NAD+ solution, empty CaP-NP). Collectively, our current study provides proof-of-concept evidence supporting the role of targeted NAD+ repletion nanotherapy in managing restenosis and improving reendothelialization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


