更正 "成人和儿童复发性多发性硬化症 II 期和 III 期试验设计及其主要终点:系统综述"。
IF 3.1
3区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
Katsutoshi Hiramatsu, Hideki Maeda.成人和儿童复发性多发性硬化症II期和III期试验设计及其主要终点:系统综述。Clin Transl Sci. 2024;17:e13794.We apologize for this error.本文章由计算机程序翻译,如有差异,请以英文原文为准。
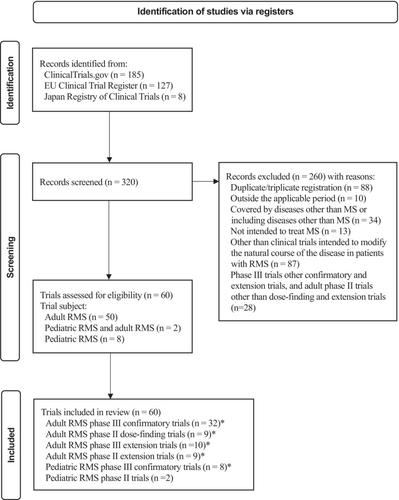
Correction to “Adult and pediatric relapsing multiple sclerosis phase II and phase III trial design and their primary endpoints: A systematic review”
Katsutoshi Hiramatsu, Hideki Maeda. Adult and pediatric relapsing multiple sclerosis phase II and phase III trial design and their primary end points: A systematic review. Clin Transl Sci. 2024;17:e13794.
We apologize for this error.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cts-Clinical and Translational Science
医学-医学:研究与实验
CiteScore
6.70
自引率
2.60%
发文量
234
审稿时长
6-12 weeks
期刊介绍:
Clinical and Translational Science (CTS), an official journal of the American Society for Clinical Pharmacology and Therapeutics, highlights original translational medicine research that helps bridge laboratory discoveries with the diagnosis and treatment of human disease. Translational medicine is a multi-faceted discipline with a focus on translational therapeutics. In a broad sense, translational medicine bridges across the discovery, development, regulation, and utilization spectrum. Research may appear as Full Articles, Brief Reports, Commentaries, Phase Forwards (clinical trials), Reviews, or Tutorials. CTS also includes invited didactic content that covers the connections between clinical pharmacology and translational medicine. Best-in-class methodologies and best practices are also welcomed as Tutorials. These additional features provide context for research articles and facilitate understanding for a wide array of individuals interested in clinical and translational science. CTS welcomes high quality, scientifically sound, original manuscripts focused on clinical pharmacology and translational science, including animal, in vitro, in silico, and clinical studies supporting the breadth of drug discovery, development, regulation and clinical use of both traditional drugs and innovative modalities.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


