将二氧化碳定向电还原为二氧化碳的高稳定性富电子镍单原子催化剂
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
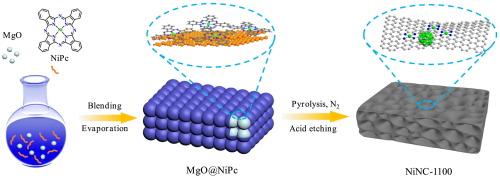
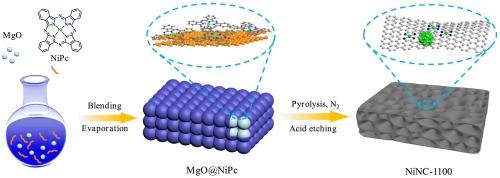
过渡金属氮碳(M-N-C)被认为是将惰性二氧化碳电化学转化为高附加值一氧化碳产品的理想候选材料。然而,以往的报道主要集中于镍单原子位点(Ni SA),而镍纳米颗粒(Ni NPs)在 CO2 电还原反应(CO2RR)中的作用却被忽视了。在此,我们结合镍酞菁热解和酸蚀策略,制备了一系列镍、N-掺杂多孔碳(NiNC-T,T 代表温度)催化剂,这些催化剂或仅含有镍单原子位点,或同时含有镍单原子位点和镍纳米粒子。值得注意的是,同时含有 Ni SAs 和 Ni NPs 的 NiNC-1100 催化剂在 -0.66 V 相对于可逆氢电极(vs. RHE)时的 CO 法拉第效率(FECO)为 99%,在较宽的电位范围(-0.66 V ∼ -1.06 V)内的 FECO 均高于 98%。此外,在连续电催化 100 小时后,NiNC-1100 的 FECO 仍保持在 95% 以上,明显优于最先进的镍单原子电催化剂。系统表征结果表明,Ni NPs 的引入可以通过增加 Ni SAs 的电子云密度来促进 CO2 的吸附和活化,从而提高 CO2RR 催化性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Highly stable and electron-rich Ni single atom catalyst for directed electroreduction of CO2 to CO
Transition metal-nitrogen-carbon (M−N−C) are considered as promising candidates for the electrochemical conversion of inert CO2 into high value-added CO products. However, previous reports have focused on Ni single-atom sites (Ni SAs) and the role of Ni nanoparticles (Ni NPs) in CO2 electroreduction reaction (CO2RR) has been overlooked. Herein, we prepared a series of Ni, N-codoped porous carbon (NiNC-T, T represents the temperature) catalysts by combining Ni phthalocyanine pyrolysis and acid etching strategy, which either contain only Ni SAs or both Ni SAs and Ni NPs. Notably, the NiNC-1100 catalyst with both Ni SAs and Ni NPs exhibited 99 % CO faradaic efficiency (FECO) at −0.66 V versus reversible hydrogen electrode (vs. RHE) and FECO above 98 % over a wide potential range (−0.66 V ∼ −1.06 V). Moreover, the FECO of NiNC-1100 remained above 95 % after 100 h of continuous electrocatalysis, which was significantly superior to that of the most advanced Ni single atom electrocatalysts. The systematic characterization results showed that the introduction of Ni NPs can promote the adsorption and activation of CO2 by increasing the electron cloud density of Ni SAs, thus enhancing the CO2RR catalytic performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


