利用红外光谱分析纳米锐钛矿和金红石颗粒表面具有单氢键供体的冰岛水分子
IF 2.8
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过分子动力学模拟和一种新型红外比值光谱法研究了二氧化钛纳米粒子的水合行为。研究发现,二氧化钛界面上的水合水含有大量单氢键供体分子,大约有五层水被限制在纳米颗粒的纳米沟槽中。据观察,这种限制效应增强了氢键的强度,并减缓了水分子在纳米粒子表面的运动。本文章由计算机程序翻译,如有差异,请以英文原文为准。
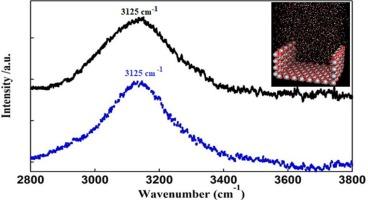
Icelike water molecules with single hydrogen bond donor on the surface of nano anatase and rutile particles by IR spectroscopy
The hydration behavior of TiO2 nanoparticles was studied by molecular dynamics simulations and a novel IR ratio spectroscopy method. It was found that the hydration water at the titanium dioxide interface contains molecules with a large number of single hydrogen bond donors, and approximately five water layers were confined within the nano-grooves of the nanoparticles. The confinement effect was observed to enhance the strength of the hydrogen bonds and to slow down the movement of the water molecules around the surface of the nanoparticles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


