通过自适应 Zn-NiOOH 亚纳米线激活和稳定晶格氧,用于氧进化反应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
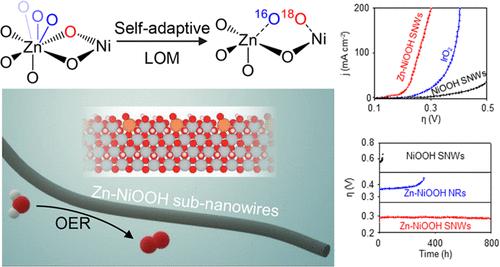
要实现水电解技术的大规模应用,高效耐用的氧进化反应催化剂至关重要。在此,我们报告了通过电化学重构 Zn-NiMoO4 SNWs 合成的新型 Zn 掺杂 NiOOH 亚纳米线(Zn-NiOOH SNWs)催化剂。Zn 的加入使 NiOOH 的氧进化反应机理从吸附剂进化机理转变为晶格氧机理,这是由于 Zn 对配位类型的适应性调整,同时也改善了反应的能量学,从而提高了稳定性和活性。此外,亚纳米线结构还进一步稳定了 Zn-NiOOH 中的晶格氧,防止其破坏性溶解。值得注意的是,Zn-NiOOH SNW 的电流密度为 10 mA cm-2,过电位仅为 179 mV,并能在 200 mA cm-2 的条件下稳定运行 800 小时,过电位变化极小。当用作碱性水电解槽的阳极时,我们的 Zn-NiOOH SNWs 催化剂在 200 mA cm-2 的水分离电流下显示出超过 500 小时的稳定性,这表明其具有良好的实际应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Activating and Stabilizing Lattice Oxygen via Self-Adaptive Zn–NiOOH Sub-Nanowires for Oxygen Evolution Reaction
Efficient and durable catalysts for the oxygen evolution reaction are essential for realizing the large-scale application of water electrolysis technologies. Here, we report a novel Zn-doped NiOOH subnanowires (Zn–NiOOH SNWs) catalyst synthesized via the electrochemical reconstruction of Zn–NiMoO4 SNWs. The inclusion of Zn triggers a transition in the oxygen evolution reaction mechanism of NiOOH from the adsorbate evolution mechanism to the lattice oxygen mechanism, resulted from Zn’s adaptive adjustment of coordination types, which also improves the reaction energetics, thereby enhancing the stability and activity. Furthermore, the subnanowire structure provides further stabilization of the lattice oxygen in Zn–NiOOH, preventing its destructive dissolution. Remarkably, Zn–NiOOH SNWs display a current density of 10 mA cm–2 with an overpotential of only 179 mV and maintain stable operation at 200 mA cm–2 for 800 h with minimal changes in overpotential, establishing them as one of the most effective catalysts involving lattice oxygen for the alkaline oxygen evolution reaction. When utilized as the anode in an alkaline water electrolyzer, our Zn–NiOOH SNWs catalyst demonstrates stability exceeding 500 h under a water-splitting current of 200 mA cm–2, indicating promising potential for practical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


