弯曲杆菌鞭毛中大型周质盘的进化使其既能高效运动又能自动凝集
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
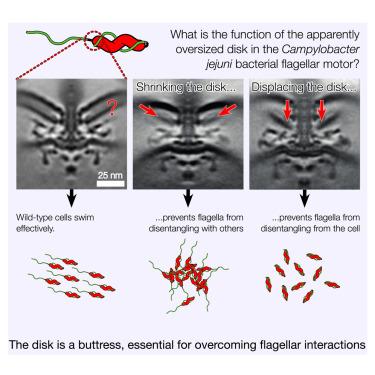
空肠弯曲杆菌(Campylobacter jejuni)和相关弯曲杆菌属(以前的epsilonproteobacteria)的鞭毛马达具有 100 nm 宽的质膜周围 "基盘",它被认为可为更宽的附加马达蛋白环提供支架以增加扭矩,但这些基盘的尺寸过大,不能仅用于为马达蛋白提供支架。在这里,我们展示了基盘是一个凸缘,在鞭毛运动的鞭毛丝与细胞体和其他丝相互作用时,它支撑着鞭毛运动。我们的研究表明,当我们缩小或移位基盘时,马达的输出不受影响,而且衰弱马达的抑制突变发生在鞭毛丝或细胞表面糖基化途径中,从而避免了需要一个凸缘来克服两个鞭毛丝之间以及鞭毛丝与细胞体之间的相互作用。我们的研究结果确定了弯曲杆菌群中鞭毛马达结构和细胞表面特性进化过程中意想不到的共同依赖性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evolution of a large periplasmic disk in Campylobacterota flagella enables both efficient motility and autoagglutination
The flagellar motors of Campylobacter jejuni (C. jejuni) and related Campylobacterota (previously epsilonproteobacteria) feature 100-nm-wide periplasmic “basal disks” that have been implicated in scaffolding a wider ring of additional motor proteins to increase torque, but the size of these disks is excessive for a role solely in scaffolding motor proteins. Here, we show that the basal disk is a flange that braces the flagellar motor during disentanglement of its flagellar filament from interactions with the cell body and other filaments. We show that motor output is unaffected when we shrink or displace the basal disk, and suppressor mutations of debilitated motors occur in flagellar-filament or cell-surface glycosylation pathways, thus sidestepping the need for a flange to overcome the interactions between two flagellar filaments and between flagellar filaments and the cell body. Our results identify unanticipated co-dependencies in the evolution of flagellar motor structure and cell-surface properties in the Campylobacterota.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


