一项对随机试验视觉摘要进行评估的横断面研究显示,报告不充分,自旋现象十分普遍。
IF 7.3
2区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
目的:可视化摘要(VAs)缺乏针对特定研究的报告指南,在医学研究传播中被越来越多地用作独立来源,尽管并非为此目的而设计。因此,我们的目标是描述:1)随机对照试验(RCT)的可视化摘要和相应的书面摘要(WAs)中报告的完整性;2)主要结果无统计学意义的 RCT 的可视化摘要和书面摘要中旋转的程度和类型(即任何可能扭曲结果解释和误导读者的报告模式):我们进行了一项横断面研究,评估了 2021 年 1 月 1 日至 2023 年 3 月 31 日期间发表的 RCT 的 VAs 和 WAs。我们通过 PubMed 在 MEDLINE 上检索了六个医学领域中影响因子最高的 15 种期刊上发表的 RCT 报告(其中有 34 种期刊发表了 RCT 的 VAs)。一位审稿人确定了发表了VA的RCT的主要报告,并从每份期刊中随机抽取了最多10份报告,以避免代表性过强。报告完整性评估基于 CONSORT 的摘要扩展。对于主要结果无统计学意义的 RCT,则采用标准化的自旋分类法对自旋进行探讨。两项评估均一式两份,如有差异,则进行讨论直至达成共识:随机抽取了 34 种期刊中的 253 篇报告。VAs中提供的信息经常不完整:分别只有47%(n=116/247)、30%(n=75/247)和35%(n=88/253)的VAs描述或显示了主要结果识别、主要结果结果和危害。WA中某些项目的报告情况略有改善,但仍不能令人满意。在主要结果不显著的试验中(n=101),57%(n=58)的自愿者协会和 55%(n=56)的自愿者工作组至少表现出一种类型的旋转。事后分析表明,与专业期刊或作者相比,影响力大的普通医学期刊编辑所撰写的VAs更完整、更准确:自愿性声明中传达的信息经常不完整、不准确,因此迫切需要参考适当的具体报告指南,以避免读者误读。本文章由计算机程序翻译,如有差异,请以英文原文为准。
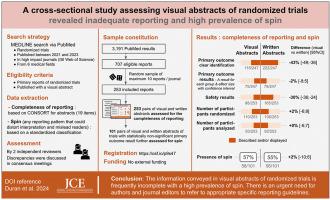
A cross-sectional study assessing visual abstracts of randomized trials revealed inadequate reporting and high prevalence of spin
Objectives
Visual abstracts (VAs) lack study-specific reporting guidelines and are increasingly used as stand-alone sources in medical research dissemination although not designed for this purpose. Therefore, our objectives were to describe 1) completeness of reporting in VAs and corresponding written abstracts (WAs) of randomized controlled trials (RCTs), and 2) the extent and type of spin (ie, any reporting pattern that could distort result interpretation and mislead readers) in VAs and WAs of RCTs with a statistically nonsignificant primary outcome.
Study Design and Setting
We conducted a cross-sectional study evaluating VAs and WAs of RCTs published between January 1, 2021, and March 3, 2023. We searched MEDLINE via PubMed for reports of RCTs published in the 15 highest impact factor journals from six medical fields (among which 34 journals producing VAs of RCTs were identified). One reviewer identified primary reports of RCTs published with a VA and randomly selected a maximum of 10 reports from each journal to avoid overrepresentation. The completeness of reporting assessment was based on the Consolidated Standards of Reporting Trials extension for abstracts. Spin was explored using a standardized spin classification for RCTs with statistically nonsignificant primary outcome results. Both assessments were conducted in duplicate, with discussion until consensus in case of discrepancy.
Results
A random sample of 253 reports from 34 journals was identified. The information provided in VAs was frequently incomplete: primary outcome identification, primary outcome results, and harms were respectively described or displayed in only 47% (n = 116/247), 30% (n = 75/247), and 35% (n = 88/253). Reporting was slightly better for some items in WAs, although still unsatisfactory. Among trials with nonsignificant primary outcome results (n = 101), 57% (n = 58) of the VAs and 55% (n = 56) of the WAs exhibited at least 1 type of spin. Posthoc analyses showed VAs produced by journal editors of high-impact general medical journals were more complete and more accurate than those produced by specialty journals or authors.
Conclusion
The information conveyed in VAs was frequently incomplete and inaccurate, highlighting the urgent need to refer to appropriate specific reporting guidelines to avoid misinterpretation by readers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Clinical Epidemiology
医学-公共卫生、环境卫生与职业卫生
CiteScore
12.00
自引率
6.90%
发文量
320
审稿时长
44 days
期刊介绍:
The Journal of Clinical Epidemiology strives to enhance the quality of clinical and patient-oriented healthcare research by advancing and applying innovative methods in conducting, presenting, synthesizing, disseminating, and translating research results into optimal clinical practice. Special emphasis is placed on training new generations of scientists and clinical practice leaders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


