工业废渣糖渣对消除结晶紫的吸附能力:实验和高级统计物理模型
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
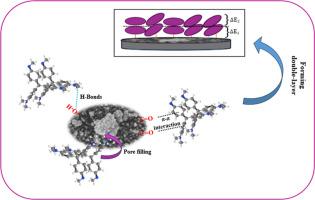
将固体工业废物回收利用为有用资源是废物管理中最关键的一环。在此,为了解毒水介质中的有毒结晶紫染料(CV),糖渣(SS)作为一种未充分利用的工业废弃物,被测试为一种有前途的吸附剂。对几个操作因素进行了优化,包括 pH 值、生物吸附剂用量、染料浓度和温度,在(pH 值 10、2 g.L-1 的 SS、10 mg.L-1 的 CV 和 25 °C)条件下,吸附量达到 24 mg.g-1。生物吸附剂的性能通过一系列研究进行了评估,包括动力学、平衡(使用传统物理模型和统计物理模型)和热力学研究。统计物理模型提供了详细的物理化学见解,具有两种能量的双层模型最准确地描述了数据,其饱和能力高达 371 mg.g-1。大多数相互作用发生在单个吸附位点(70%),其余 30% 发生在两个位点,涉及平行和非平行取向。SS 表面的介孔结构为 CV 吸附提供了最佳尺寸。孔隙填充、范德华力、氢键、π-π 和静电相互作用被认为是 CV-SS 系统的可能机制。热力学测量结果表明,CV 吸附是自发的(ΔG° <0),ΔH° 是放热的(- 41.390 kJ mol-1)。总之,这项研究为推广生态水处理策略做出了重大贡献,突出了使用固态金属作为水污染物修复的环境替代品的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adsorption ability of sugar scum as industrial waste for crystal violet elimination: Experimental and advanced statistical physics modeling
Recycling solid industrial waste into useful resources is the most critical aspect of managing waste. Herein, for detoxifying poisonous crystal violet dye (CV) in aqueous media, sugar scum (SS) as an underutilized industrial discard has been tested as a promising adsorbent.
The SS was thoroughly analyzed beforehand and after the adsorption process through various characterization techniques. Several operational factors, including pH, biosorbent dosage, dye concentration, and temperature, were optimized, reaching 24 mg.g-1 at (pH 10, 2 g.L−1 of SS, 10 mg.L−1 of CV and 25 °C). The bioadsorbent's performance was assessed through a range of studies involving kinetic, equilibrium (using both conventional and statistical physics models), and thermodynamic investigations.
Based on the results, the equilibrium curves best fit the Freundlich model, indicating that multilayer adsorption occurs on a heterogeneous active sites surface. The statistical physics models provided detailed physiochemical insights, the double-layer with two energies model was most accurately describing the data, with a high saturation capability of 371 mg.g−1. Most interactions occur at a single adsorption site (70 %), with the remaining 30 % at two sites, involving both parallel and non-parallel orientations. The mesoporous structure of the SS surface provides an optimum size for CV adsorption. Pore filling, Van der Waals forces, hydrogen bonding, π-π and electrostatic interactions are proposed as the possible mechanisms in the CV-SS system. Thermodynamic measurements indicate that CV adsorption is spontaneous (ΔG° < 0) and exothermic ΔH° (− 41.390 kJ mol−1).
The SS recyclability was assessed, exhibiting encouraging sustainability with a slight fall in effectiveness (∼7 %) after 5 sequential usages. Altogether, this study makes a substantial contribution to the promotion of ecological water treatment strategies, which highlights the utility of using SS as an environmental alternative to water contaminant remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


