亲水性吸附剂上 Al3+ 离子与水的界面动力学:分子动力学研究
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
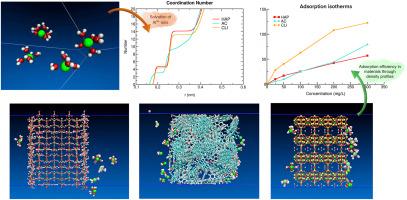
阳极氧化工业产生的残留物含有 Al3+ 等金属离子,这些污染物在水体中的存在对生态和人类健康造成了严重危害。重金属离子的吸附是一种广泛使用的方法,因为它是一种易于实施、生态友好和具有成本效益的工艺。这项研究报告了不同表面对 Al3+ 离子吸附过程的影响,利用分子动力学模拟阐明了离子的吸附机理。通过计算研究了三种吸附剂的模型:羟基磷灰石(HAP)、活性炭(AC)和clinoptilolite(CLI),以确定最合适的吸附剂。通过计算吸附等温线,对三种不同吸附材料从水溶液中去除 Al3+ 离子的效果进行了评估。研究结果与骨炭对 Al3+ 的吸附实验数据进行了比较,结果表明 HAP 模型与实验数据具有良好的定性相似性。模拟结果和实验数据与 Freundlich 模型的拟合效果令人满意,拟合参数显示模拟数据和实验数据之间的差异小于 3%。对于三种吸附剂,模拟数据与 Freundlich 等温线吸附模型的拟合程度较高,这表明每种吸附剂在吸附容量不同的表面上都存在异质吸附。为了研究吸附机理,计算了密度曲线、RDF、H 键数量、分子最小距离和分子间相互作用。吸附剂表面的亲水性或两亲性以及溶液中离子的溶解结构对 Al3+ 离子的吸附效率影响很大。在每个体系中都会出现溶解现象,但只有 AC 吸附剂的溶解程度低于 CLI 和 HAP 表面。CLI 模型的吸附能力最高,这是因为它具有强烈的亲水性,使吸附在其表面的水分子具有更强的亲和力和稳定性,因此可以吸附更多的 Al3+ 离子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interfacial dynamics of Al3+ ions and water on hydrophilic adsorbents: A molecular dynamics study
The residues generated from the anodizing industry contain metallic ions like Al3+, and the presence of these pollutants in water bodies cause serious concern because of the ecological and human health risks. The adsorption of heavy metal ions is a widely used option due to it is an easy to implement, eco-friendly, and cost-effective process. This work reports the effect of different surfaces on the adsorption process of Al3+ ions to clarify the adsorption mechanism of the ions using molecular dynamics simulations. The models of three adsorbents: hydroxyapatite (HAP), an activated carbon (AC), and clinoptilolite (CLI) were computationally investigated to identify the most suitable adsorbent. The calculated adsorption isotherms allowed the evaluation of three different adsorbent materials for the removal of Al3+ ions from an aqueous solution. The findings were compared with experimental data of Al3+ adsorption onto bone char and a good qualitative similarity with experimental data was obtained for the HAP model. The results obtained by simulation as well as the experimental data fit satisfactorily to the Freundlich model, the fitting parameters show differences less than 3 % between the simulated and experimental data. For the three adsorbents, the simulated data fit better to the Freundlich isotherm adsorption model, suggesting heterogeneous adsorption on surfaces with variable adsorption capacity in each case. To study the adsorption mechanism, density profiles, RDFs, number of H-bonds, molecular minimum distances and intermolecular interactions were calculated. The efficiency of Al3+ ion adsorption is strongly influenced by the hydrophilic or amphiphilic nature of the adsorbent surface, as well as by the solvation structure of the ions in the solution. In each system, the solvation phenomenon occurs, although only the AC adsorbent exhibits it to a lesser extent compared to CLI and HAP surfaces. The CLI model had the highest adsorption capacity, due to its intense hydrophilic behavior, which leads to greater affinity and stability in the water molecules adsorbed on its surface and, therefore, allows a greater adsorption of Al3+ ions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


