埃博瑞他单抗治疗复发/难治性大B细胞淋巴瘤:EPCORE NHL-1关键试验的两年随访结果
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
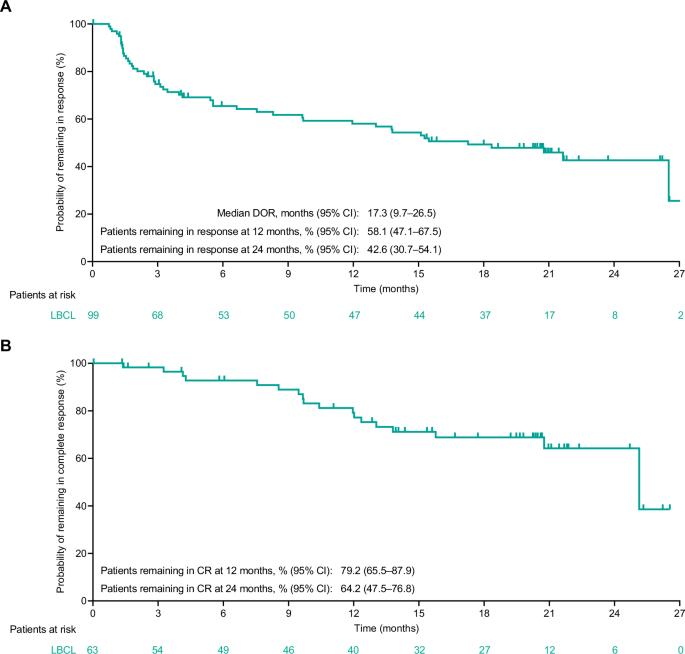
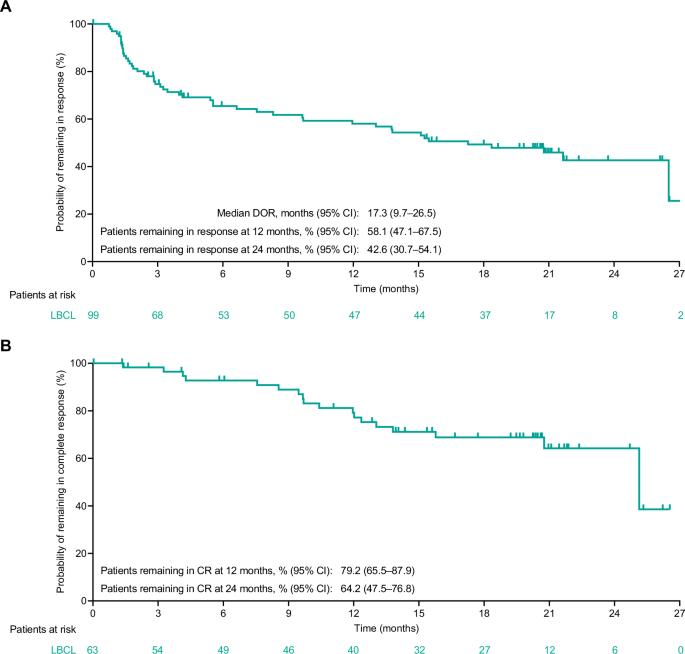
针对复发或难治性(R/R)大 B 细胞淋巴瘤(LBCL)的关键性 EPCORE® NHL-1 研究的初步结果(中位随访 10.7 个月)显示,CD3xCD20 双特异性抗体 epcoritamab 作为单药治疗时能产生深远而持久的反应。我们报告了 LBCL 患者的长期疗效和安全性结果(N = 157;中位随访 25.1 个月)。截至2023年4月21日,总反应率为63.1%,完全反应(CR)率为40.1%。估计24个月的无进展生存期(PFS)和总生存期(OS)分别为27.8%和44.6%。估计有 64.2% 的完全应答者在 24 个月后仍处于 CR 状态。在完全应答者中,估计24个月的PFS和OS率分别为65.1%和78.2%。在119例有极小残留病(MRD)价值的患者中,45.4%的患者MRD阴性,这与较长的PFS和OS相关。各预定义亚组的 CR 率基本一致:36% 曾接受过嵌合抗原受体 (CAR) T 细胞治疗,32% 患有原发性难治性疾病,37% 国际预后指数≥3。最常见的治疗突发不良事件是细胞因子释放综合征(51.0%)、热病(24.8%)、疲劳(24.2%)和中性粒细胞减少(23.6%)。这些结果凸显了艾普科瑞他单抗治疗R/R LBCL的长期疗效,在各个亚组都能产生深度反应,包括难以治疗的疾病和预期预后不良的患者(ClinicalTrials.gov注册:NCT03625037)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial
Primary results (median follow-up, 10.7 months) from the pivotal EPCORE® NHL-1 study in relapsed or refractory (R/R) large B-cell lymphoma (LBCL) demonstrated deep, durable responses with epcoritamab, a CD3xCD20 bispecific antibody, when used as monotherapy. We report long-term efficacy and safety results in patients with LBCL (N = 157; 25.1-month median follow-up). As of April 21, 2023, overall response rate was 63.1% and complete response (CR) rate was 40.1%. Estimated 24-month progression-free survival (PFS) and overall survival (OS) rates were 27.8% and 44.6%, respectively. An estimated 64.2% of complete responders remained in CR at 24 months. Estimated 24-month PFS and OS rates among complete responders were 65.1% and 78.2%, respectively. Of 119 minimal residual disease (MRD)-evaluable patients, 45.4% had MRD negativity, which correlated with longer PFS and OS. CR rates were generally consistent across predefined subgroups: 36% prior chimeric antigen receptor (CAR) T-cell therapy, 32% primary refractory disease, and 37% International Prognostic Index ≥3. The most common treatment-emergent adverse events were cytokine release syndrome (51.0%), pyrexia (24.8%), fatigue (24.2%), and neutropenia (23.6%). These results underscore the long-term benefit of epcoritamab for treating R/R LBCL with deep responses across subgroups, including patients with hard-to-treat disease and expected poor prognosis (ClinicalTrials.gov Registration: NCT03625037).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


