外用药物产品在不断变化的皮肤病领域中的机遇。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
尽管皮肤病的发病率很高,对生活质量的影响也很大,但治疗这类疾病的药物产品却很少成为畅销书。由于人们普遍认为皮肤病药物产品的商业潜力有限,因此限制了创新,并鼓励采用更为保守的开发方法。例如,当时的重点是对现有的活性药物成分(API)进行再利用/再改造,专门用于局部给药途径。然而,如今的情况已截然不同,部分原因是美国食品药品管理局(US FDA)于 2017 年批准了首个治疗特应性皮炎的单克隆抗体药物 Dupixent(dupilimab),这在一定程度上催化了 Dupixent 的大获成功。Dupixent 的成功不仅鼓励了其他生物制剂的开发,还激发了新型皮肤药物产品的(再)开发,这些产品可以获得局部用药的诸多益处。我们还目睹了开发工作(及相关风险)向中小型制药公司外包的转变,这些公司通常需要合同研究与开发/生产组织(CRO 和 CDMO)的支持。这种趋势也强调了在外用制剂设计方面需要更多的专业知识,以及相关的商业和监管考虑因素。如今,我们相信外用药物产品不仅是皮肤病治疗的重要组成部分,而且在商业上也是可行的。在这篇观点文章中,我们将探讨在当前市场环境下采取协调一致的开发战略所面临的挑战和机遇、外用药物产品的制剂创新以及促进合理外用药物制剂开发的技术进步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
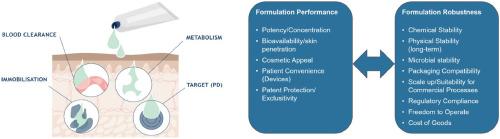
Opportunities of topical drug products in a changing dermatological landscape
Despite the prevalence and the impact on quality of life of dermatological indications, drug products to treat such conditions have rarely been blockbusters. The prevailing perception of a limited commercial potential of dermatological drug products has restricted innovation and encouraged a more conservative development approach. For example, the focus was on repurposing/reformulation of existing active pharmaceutical ingredients (APIs) specifically for the topical delivery route.
However, the situation is quite different today catalyzed in part by the blockbuster success of Dupixent (dupilumab), the first monoclonal antibody treatment for atopic dermatitis which has been approved by the US Food and Drug Administration (US FDA) in 2017. Dupixent's success not only encouraged the development of other biologics but also inspired the (re-)development of new dermal drug products that can reap the many benefits of topical administration.
We have also witnessed a shift toward outsourcing development efforts (and associated risks) towards small- to mid-size pharmaceutical companies which often require support of contract research and development/manufacturing organizations (CRO and CDMO). Such trends also emphasize the need of greater expertise in topical formulation design, as well as associated commercial and regulatory considerations.
Today, we believe that topical drug products remain not only an essential but also commercially viable class of dermatological therapeutics. In this opinion article, we will address the challenges as well as opportunities of coherent development strategies in the current market environment, formulation innovations of topical drug products and technological advances to facilitate rational topical drug formulation development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


