移植人胎盘间充质干细胞可修复肺泡上皮屏障,缓解脂多糖诱发的急性肺损伤。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
急性肺损伤(ALI)和急性呼吸窘迫综合征(ARDS)的死亡率很高,而有效的治疗方法却很少。移植人胎盘间充质干细胞(hPMSCs)可减轻急性肺损伤,但其机制尚不清楚。我们的研究旨在阐明人胎盘间充质干细胞(hPMSCs)对脂多糖(LPS)诱导的ALI的潜在保护作用和治疗机制。使用 hPMSCs 治疗可改善肺组织病理学损伤,降低肺损伤评分,减少支气管肺泡灌洗液(BALF)中的白细胞数量和蛋白质水平,保护受损的肺泡上皮屏障、并逆转了 LPS 诱导的白细胞介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)在 BALF 中的上调,以及抗炎因子白细胞介素-6(IL-10)的下调。此外,给 ALI 小鼠注射 hPMSCs 可抑制血管紧张素(Ang)II 的激活,促进 ACE2 和 Ang (1-7) 的表达水平。ACE2基因缺失后,ALI小鼠的病理损伤、炎症水平和肺泡上皮屏障破坏程度升高,肾素血管紧张素系统(RAS)失衡加剧。在 ACE2 KO 小鼠中,hPMSCs 的治疗效果明显降低。我们的研究结果表明,ACE2 在 hPMSCs 修复肺泡上皮屏障以防止 ALI 中发挥了关键作用,为 ALI 的临床治疗奠定了新的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
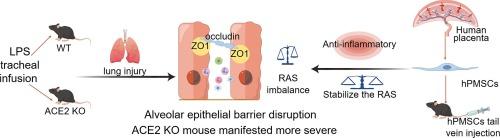
Human placental mesenchymal stem cells transplantation repairs the alveolar epithelial barrier to alleviate lipopolysaccharides-induced acute lung injury
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are accompanied by high mortality rates and few effective treatments. Transplantation of human placental mesenchymal stem cells (hPMSCs) may attenuate ALI and the mechanism is still unclear. Our study aimed to elucidate the potential protective effect and therapeutic mechanism of hPMSCs against lipopolysaccharide (LPS)-induced ALI, An ALI model was induced by tracheal instillation of LPS into wild-type (WT) and angiotensin-converting enzyme 2 (ACE2) knockout (KO) male mice, followed by injection of hPMSCs by tail vein. Treatment with hPMSCs improved pulmonary histopathological injury, reduced pulmonary injury scores, decreased leukocyte count and protein levels in bronchoalveolar lavage fluid(BALF), protected the damaged alveolar epithelial barrier, and reversed LPS-induced upregulation of pro-inflammatory factors Interleukin-6 (IL-6) and Tumor necrosis factor-α(TNF-α) and downregulation of anti-inflammatory factor Interleukin-6(IL-10) in BALF. Moreover, administration of hPMSCs inhibited Angiotensin (Ang)II activation and promoted the expression levels of ACE2 and Ang (1–7) in ALI mice. Pathological damage, inflammation levels, and disruption of alveolar epithelial barrier in ALI mice were elevated after the deletion of ACE2 gene, and the Renin angiotensin system (RAS) imbalance was exacerbated. The therapeutic effect of hPMSCs was significantly reduced in ACE2 KO mice. Our findings suggest that ACE2 plays a key role in hPMSCs repairing the alveolar epithelial barrier to protect against ALI, laying a new foundation for the clinical treatment of ALI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


