通过拓扑控制π共轭实现从二元杂环到四元杂环的飞跃
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这项研究中,我们探讨了基于 2,2′-(5,11-二氢吲哚并[3,2-b]咔唑-3,9-二基)二丙二腈(以下简称 PH)的二烷基(id)系列。PH 的中心苯环(CB)上的氢原子被一系列具有不同长度的 π 共轭链、电子供体或电子吸附性的取代基所取代,以研究它们如何改变母体化合物的二叉性质。通过在 PH 的 CB 环两侧的对位相对位置上双键连接两个基团,分子的二元性增加了 88-89%。这打破了产生两个自由基中心的未配对电子之间的直接 π 键合,并将化合物的最小多辐射特性限制为二拉德。我们的研究表明,只要有足够的芳香稳定性,就可以设计出二元和四元化合物,并且可以通过控制 π 共轭的拓扑结构来调节它们的多辐射特性。因此,我们在二拉基和四拉基之间架起了一座桥梁,并由此产生了四拉基(oid)分子,我们在最近的信中预测这种分子具有狭窄的从低自旋到高自旋的能隙。本文章由计算机程序翻译,如有差异,请以英文原文为准。
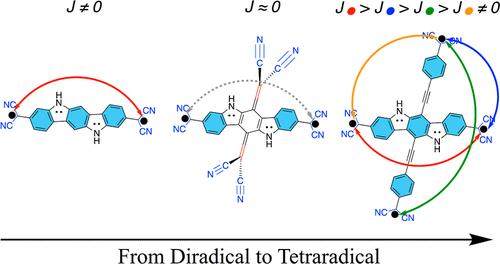
Leap from Diradicals to Tetraradicals by Topological Control of π-Conjugation
In this work, we explore the series of diradical(oid)s based on 2,2′-(5,11-dihydroindolo[3,2-b]carbazole-3,9-diyl)dimalononitrile (further referred to as PH). Hydrogen atoms in the central benzenoid (CB) ring of PH are substituted by the series of substituents with various lengths of π-conjugated chain and electron-donating or electron-withdrawing properties to study how they modulate the diradical character of the parent compound. The diradical character of molecules increases up to 88–89% by two groups doubly bonded to both sides of the CB ring of PH in para relative positions. This breaks the direct π-conjugation between unpaired electrons that gives rise to two radical centers and restricts the minimal polyradical identity of the compound to diradical. We show that diradicals and tetraradicals can be designed, and their polyradical character can be modulated by controlling the topology of π-conjugation as long as there is sufficient aromatic stabilization. Henceforth, the bridge between diradicals and tetraradicals is established, leading to the tetraradical(oid) molecule, which has been predicted to have narrow low-spin to high-spin energy gaps in our recent Letter.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


