通过镍催化 1,2-Glycosyl Orthoesters 和乙烯基卤化物的交叉亲电偶联立体选择性合成 C-乙烯基糖苷
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
综合摘要 本研究开发了一种高度立体选择性的镍催化交叉亲电偶联方法,可将容易获得的、新型的、稳定的氧基糖基自由基前体,特别是 1,2-糖基原酯偶联起来。这种方法为合成多种 C-乙烯基糖苷提供了有效途径,其特点是产率高、立体选择性好、反应条件温和、底物范围广以及所得产物可进行多种转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
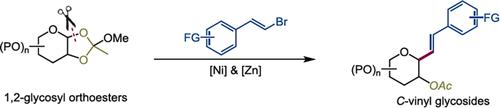
Stereoselective Synthesis of C-Vinyl Glycosides via Nickel-Catalyzed Cross-Electrophile Couplings of 1,2-Glycosyl Orthoesters and Vinyl Halides
A highly stereoselective nickel-catalyzed cross-electrophile coupling of readily accessible, novel, stable oxygen-based glycosyl radical precursors, specifically 1,2-glycosyl orthoesters, is developed. This approach offers an effective pathway to synthesize diverse C-vinyl glycosides, characterized by good yields, excellent stereoselectivity, mild reaction conditions, a broad substrate scope, and versatile transformations of the resulting products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


