无酰基埃辛衍生物的合成研究
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
综合摘要以原茜草素为起始原料,通过协调应用Yu和Schmidt糖基化协议,首次实现了无酰基茜草素衍生物的合成。由于苷元上存在酰基而产生的非特异性毒性阻碍了萃取物的广泛应用,因此,针对无酰基原芹菜甙元型皂苷的既定策略将大大简化获得无酰基萃取物衍生物的过程,从而加快这些珍贵化合物的医药应用步伐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
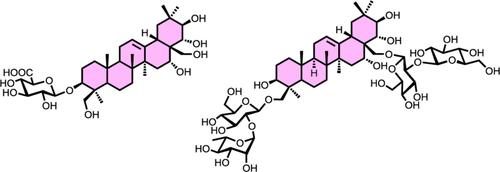
Synthetic Investigation toward Acyl Group-Free Escin Derivatives
With protoescigenin as starting material and through orchestrated application of Yu and Schmidt glycosylation protocols, the synthesis of acyl group-free escin derivatives was achieved for the first time. As the undesired non-specific toxicity, originating from the existence of acyl groups on aglycone, prohibits the wide application of escins, the established strategies toward non-acylated protoescigenin-type saponins would dramatically ease the access to escin derivatives dispense of acyl groups, thereby speeding up the pace of pharmaceutical use of these valuable compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


