对 "制备以氮化石墨碳为支撑的介孔 CrTe 作为水氧化的高效电催化剂 "的更正
IF 3.7
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
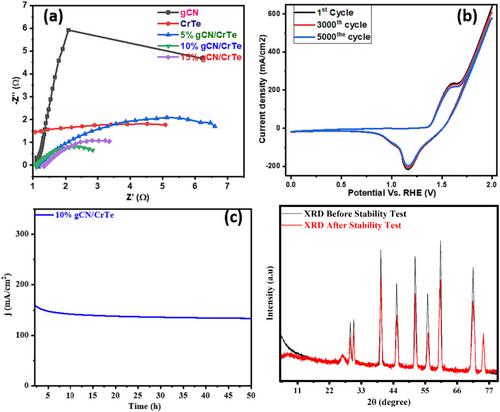
Seliem, AF, Ashiq, MF, Jabbour, K, Mohammed, AYA, Bano, N, Attia, A, Ansari, MN, Ashiq, MN, Ibrahim, MM.在氮化石墨碳上制备介孔 CrTe 作为高效水氧化电催化剂。Appl Organomet Chem.2023; 37(11):e7240.我们注意到图 2 中的图表经过了平滑处理和基线校正。在此过程中,学生混淆了一些数据。这给尊敬的期刊读者造成了困惑和误解。现在,我们在修订后的图 2 中展示了未经平滑和基线校正的数据,从而提高了权威期刊读者的理解能力。图 2.gCN、CrTe、5% gCN/CrTe、10% gCN/CrTe 和 15% gCN/CrTe 纳米复合材料的傅立叶变换红外光谱。对于图 8d,我们对稳定后的 XRD 光谱进行了校正,由于基线校正和平滑数据以去除噪声,该光谱特别容易混淆。我们提供了未经处理的 XRD 图谱,以便期刊读者更清楚地理解。图 8:(a) gCN、CrTe、10% gCN/CrTe 和 15% gCN/CrTe 纳米复合材料的 EIS Nyquist 图;(b) CV 循环;(c) 时变测量法;(d) 10% gCN/CrTe 稳定性测试前后的 XRD 图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Correction to “Fabrication of mesoporous CrTe supported on graphitic carbon nitride as an efficient electrocatalyst for water oxidation”
Seliem, AF, Ashiq, MF, Jabbour, K, Mohammed, AYA, Bano, N, Attia, A, Ansari, MN, Ashiq, MN, Ibrahim, MM. Fabrication of mesoporous CrTe supported on graphitic carbon nitride as an efficient electrocatalyst for water oxidation. Appl Organomet Chem. 2023; 37(11):e7240.
Figure 2. FTIR spectra of gCN, CrTe, 5% gCN/CrTe, 10% gCN/CrTe, and 15% gCN/CrTe nanocomposite.
Figure 8. (a) EIS Nyquist plot of gCN, CrTe, 10% gCN/CrTe, and 15% gCN/CrTe nanocomposite, (b) CV cycles, (c) chronoamperometry, and (d) XRD pattern before and after stability test of 10% gCN/CrTe.
We apologize for these errors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Organometallic Chemistry
化学-无机化学与核化学
CiteScore
7.80
自引率
10.30%
发文量
408
审稿时长
2.2 months
期刊介绍:
All new compounds should be satisfactorily identified and proof of their structure given according to generally accepted standards. Structural reports, such as papers exclusively dealing with synthesis and characterization, analytical techniques, or X-ray diffraction studies of metal-organic or organometallic compounds will not be considered. The editors reserve the right to refuse without peer review any manuscript that does not comply with the aims and scope of the journal. Applied Organometallic Chemistry publishes Full Papers, Reviews, Mini Reviews and Communications of scientific research in all areas of organometallic and metal-organic chemistry involving main group metals, transition metals, lanthanides and actinides. All contributions should contain an explicit application of novel compounds, for instance in materials science, nano science, catalysis, chemical vapour deposition, metal-mediated organic synthesis, polymers, bio-organometallics, metallo-therapy, metallo-diagnostics and medicine. Reviews of books covering aspects of the fields of focus are also published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


