跳出能量框思考:利用网状化学稳定和绿化高能材料
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
网状化学为通过调整构件系统地设计具有不同孔隙的多孔材料提供了有趣的机会。其中,框架材料在设计新型功能材料(包括高能材料)方面表现出色,可广泛应用于各个领域。高能材料广泛应用于火箭、卫星、采矿和隧道等领域。就高能材料而言,爆炸体和富氮杂环是高能化合物的基本组成单元。然而,在分子水平合成高能量密度材料(HEDMs)的传统策略面临着平衡能量和稳定性的长期挑战。受网状化学的启发,富氮杂环为设计多样化的配位相互作用提供了多种氮位点。离子键相互作用存在于各种高能盐中。此外,大多数可转移的爆炸物,如硝基、硝氨基和氨基,都能形成强大的氢键网络。基于这些非共价相互作用(如配位、离子和/或氢键 (HB))和/或共价相互作用可决定高能燃料和氧化剂成分的分子间堆积/连接,网状化学提供了一个从单分子设计发展到各种高能框架(具有优异综合性能的高能框架的 E)的新平台。例如,为了实现与金属的配位或引入足够的氢键供体/受体结构单元,高能框架材料的宿主结构通常含有较少的富氧取代基(如硝基),因此框架的宿主分子(F)在晶体水平上可以提高高能框架的综合稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
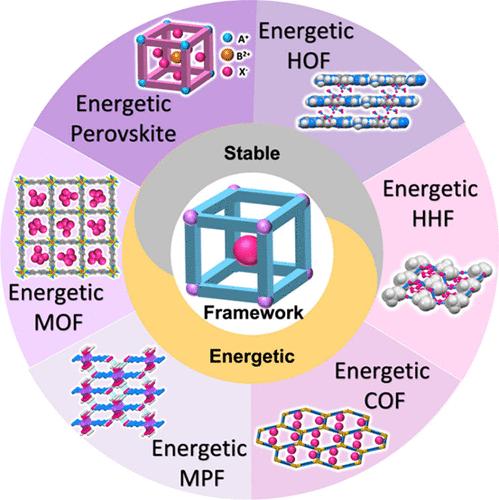
Thinking Outside the Energetic Box: Stabilizing and Greening High-Energy Materials with Reticular Chemistry
Reticular chemistry has provided intriguing opportunities for systematically designing porous materials with different pores by adjusting the building blocks. Among them, framework materials have demonstrated outstanding performance for the design of new functional materials used in a broad range of fields, including energetic materials. Energetic materials are widely used for rockets, satellites, mining, and tunneling. In terms of energetic materials, explosophores and nitrogen-rich heterocycles are fundamental building blocks for high-energy compounds. However, the traditional strategy of synthesizing HEDMs (high energy density materials) at the molecular level has faced the long-term challenge of balancing energy and stability. Inspired by reticular chemistry, nitrogen-rich heterocycles offer diverse nitrogen sites for designing diversified coordination interactions. Ionic bond interactions exist in a wide range of energetic salts. Furthermore, most metastable explosophores, e.g., nitro, nitramino, and amino groups, can form strong hydrogen-bonding networks. Based on these noncovalent interactions (such as coordination, ionic, and/or hydrogen bonds (HBs)) and/or covalent interactions can determine intermolecular packing/linkage of the energetic fuel and oxidizer components, reticular chemistry provides a new platform evolving from single-molecular design to various energetic frameworks (E of the energetic frameworks with superior comprehensive properties. For example, to achieve coordination with metals or introduce sufficient hydrogen bond donor/acceptor structural units, the host structure of energetic framework materials usually contains less oxygen-rich substituents such as nitro, so the host molecules of the framework, F) at the crystal level, which can enhance the integrated stabilities of EFs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


