利用水电解产生的 H2,在连续闭环流动系统中进行黄素依赖性烯还原的 H2 驱动生物催化反应。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管对高效和可持续化学工艺的需求日益增长,但利用生物催化技术开发用于精细化学品生产的可扩展系统仍是一项重大挑战。我们开发了一种可扩展的流动系统,利用固定化酶促进黄素依赖性生物催化,并将其作为不对称烯还原的概念验证。该系统集成了依赖黄素的老黄酶(OYE)和可溶性氢酶,以实现 OYE 辅因子 FMNH2 的氢驱动再生。分子氢是利用质子交换膜(PEM)电解槽通过水电解产生的,并通过设计的气体膜添加模块以高扩散率引入流动系统。该流动系统显示出卓越的稳定性和可重复使用性,酮异佛尔酮到左旋二酮的转化率始终大于 99%。在还原各种环状烯酮时,它还表现出了多功能性和选择性,并可进一步扩展到基于黄素的生物催化方法和气体依赖性反应。因此,这种电驱动连续流系统在推进精细化学合成的可持续工艺方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
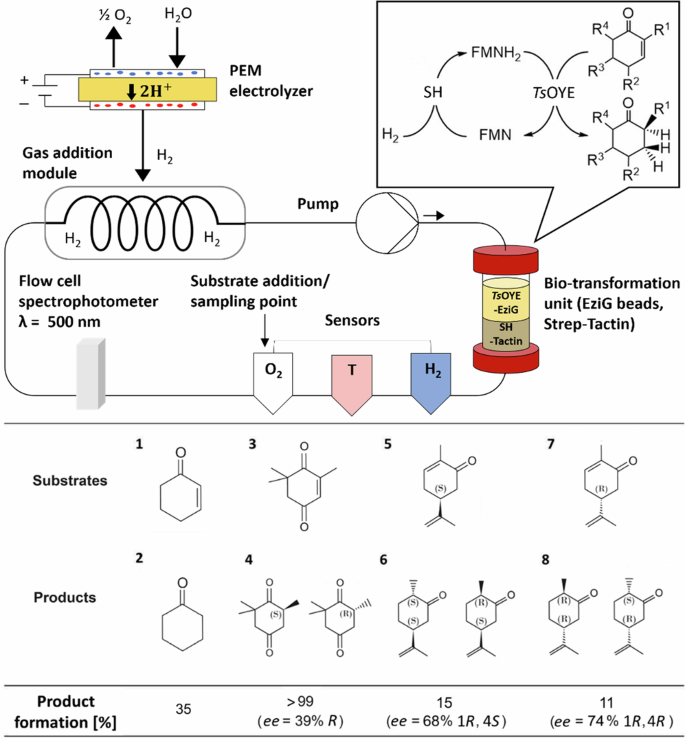
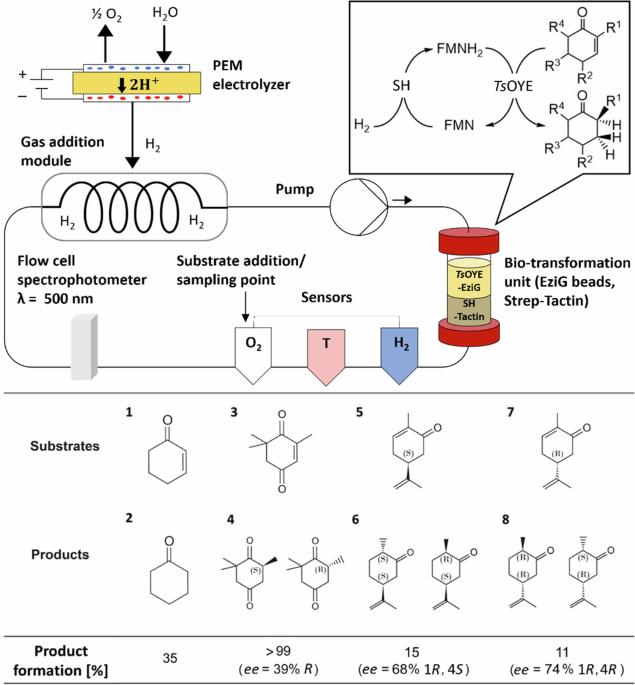
H2-driven biocatalysis for flavin-dependent ene-reduction in a continuous closed-loop flow system utilizing H2 from water electrolysis
Despite the increasing demand for efficient and sustainable chemical processes, the development of scalable systems using biocatalysis for fine chemical production remains a significant challenge. We have developed a scalable flow system using immobilized enzymes to facilitate flavin-dependent biocatalysis, targeting as a proof-of-concept asymmetric alkene reduction. The system integrates a flavin-dependent Old Yellow Enzyme (OYE) and a soluble hydrogenase to enable H2-driven regeneration of the OYE cofactor FMNH2. Molecular hydrogen was produced by water electrolysis using a proton exchange membrane (PEM) electrolyzer and introduced into the flow system via a designed gas membrane addition module at a high diffusion rate. The flow system shows remarkable stability and reusability, consistently achieving >99% conversion of ketoisophorone to levodione. It also demonstrates versatility and selectivity in reducing various cyclic enones and can be extended to further flavin-based biocatalytic approaches and gas-dependent reactions. This electro-driven continuous flow system, therefore, has significant potential for advancing sustainable processes in fine chemical synthesis. Flavin-based biocatalysis using flavin mononucleotide (FMN) cofactor attracts significant attention for its application in asymmetric alkene reduction and various other reactions, however, the scale-up of flavin-based biocatalysis in flow remains unexplored. Here, the authors develop a closed-loop flow platform for H2-driven regeneration of cofactor FMNH2 and ene-reduction using immobilized Old Yellow Enzyme, achieving >99% conversion of ketoisophorone to levodione.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


