拟南芥叶球成员与寄主基因型和干旱的大陆尺度关系
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
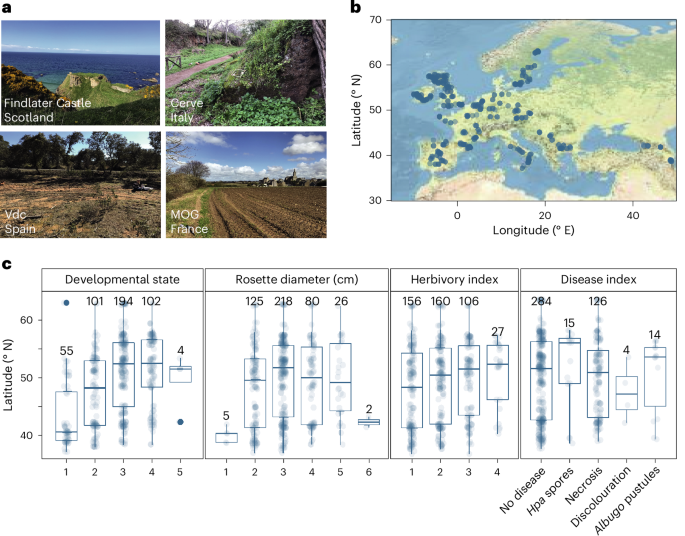
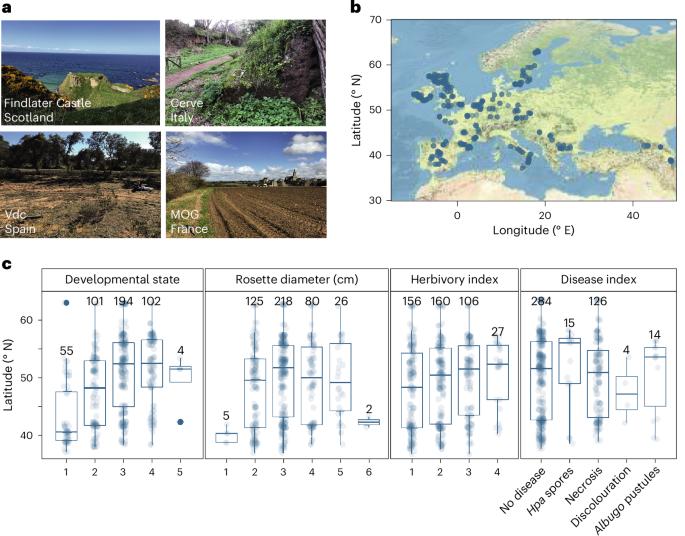
在全球不同地区,植物被不同的病原微生物群和共生微生物群定植,但驱动其地理变异的因素在很大程度上是未知的。在这里,我们利用 16S 核糖体 DNA 和枪式测序技术,对拟南芥叶片微生物组与宿主遗传学和气候变量之间的关系进行了表征,这些微生物组来自拟南芥原产地欧洲的 267 个种群。通过比较 575 个主要细菌扩增子变体(系统型)的分布,我们发现拟南芥叶片微生物组的组成是沿着纬度梯度分离的。微生物组组成的纬度梯度是由干旱指标以及宿主的空间遗传学预测的。为了验证干旱和宿主基因型的相对影响,我们进行了一项普通花园田间研究,发现10%的核心细菌直接受干旱影响,20%的核心细菌受宿主基因与干旱相关性的影响。这些数据为植物微生物组领域提供了宝贵的资源,已确定的关联表明,干旱可以通过叶片微生物组直接或间接地影响连翘的遗传变异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Continental-scale associations of Arabidopsis thaliana phyllosphere members with host genotype and drought
Plants are colonized by distinct pathogenic and commensal microbiomes across different regions of the globe, but the factors driving their geographic variation are largely unknown. Here, using 16S ribosomal DNA and shotgun sequencing, we characterized the associations of the Arabidopsis thaliana leaf microbiome with host genetics and climate variables from 267 populations in the species’ native range across Europe. Comparing the distribution of the 575 major bacterial amplicon variants (phylotypes), we discovered that microbiome composition in A. thaliana segregates along a latitudinal gradient. The latitudinal clines in microbiome composition are predicted by metrics of drought, but also by the spatial genetics of the host. To validate the relative effects of drought and host genotype we conducted a common garden field study, finding 10% of the core bacteria to be affected directly by drought and 20% to be affected by host genetic associations with drought. These data provide a valuable resource for the plant microbiome field, with the identified associations suggesting that drought can directly and indirectly shape genetic variation in A. thaliana via the leaf microbiome. The leaf microbiome compositions of 267 Arabidopsis thaliana populations across Europe reveal associations with climate and plant genetics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


