烯丙基二氟化物的光催化脱氟α-氨基烷基化反应。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-04
DOI:10.1021/acs.joc.4c01861
引用次数: 0
摘要
我们设计了一种光催化工艺,通过烯丙基二氟化物的脱氟官能化合成单氟烯烃。以 N-烷基苯胺为α-氨基烷基自由基的前体,α-氨基烷基自由基与烯丙基二氟化物发生区域选择性加成,随后进行 SET 和氟消除,生成一氟烯烃。在苯胺上形成的 C-C 键对取代程度最低的碳 α 至氮具有位点选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
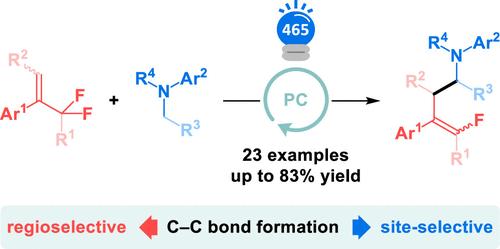
Photocatalytic Defluorinative α-Aminoalkylation of Allylic Difluorides.
A photocatalytic process was devised to synthesize monofluoroalkenes via defluorinative functionalization of allylic difluorides. N-Alkylanilines are used as precursors to α-aminoalkyl radicals, which undergo regioselective addition to allylic difluorides, and subsequent SET and fluoride elimination produce monofluoroalkenes. C-C bond formation on the aniline is site-selective for the least substituted carbon α to nitrogen.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


