通过 C-S 键活化实现苄基锍盐与硫代磺酸盐的交电偶联。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-03
DOI:10.1021/acs.joc.4c01786
引用次数: 0
摘要
通过 C-S 键裂解,实现了锌介导的苄基锍盐与硫代磺酸盐的交叉亲电偶联。在不含过渡金属的条件下,还原硫代反应进行顺利,以良好的产率获得了所需的苄基硫化物,并表现出广泛的底物范围和良好的官能度耐受性。此外,该反应还可应用于硒磺酸盐作为有效的硒化剂,并可进行放大合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
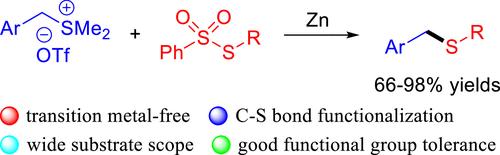
Cross-Electrophile Couplings of Benzyl Sulfonium Salts with Thiosulfonates via C-S Bond Activation.
A zinc-mediated cross-electrophile coupling of benzyl sulfonium salts with thiosulfonates via C-S bond cleavage was achieved. The reductive thiolation proceeded well under transition metal-free conditions to afford the desired benzyl sulfides in good yields, exhibiting both broad substrate scope and good functionality tolerance. In addition, the reaction could be applied to the use of selenosulfonate as an effective selenylation agent and be subjected to scale-up synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


