可切除的局部晚期食管鳞状细胞癌新辅助康瑞珠单抗加化疗的临床疗效和免疫反应:2 期试验。
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
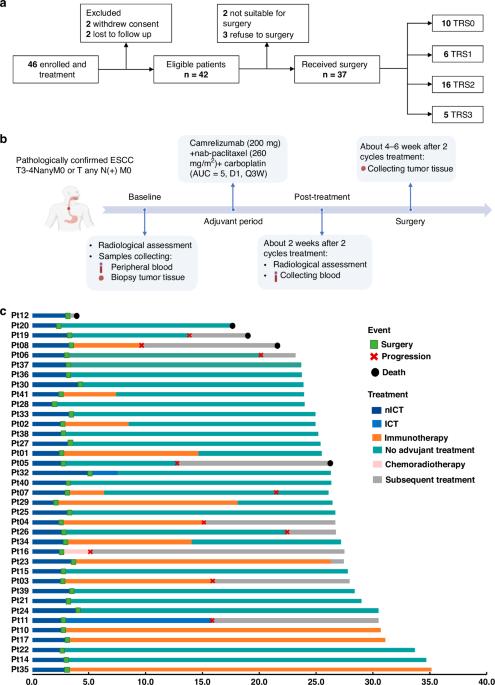
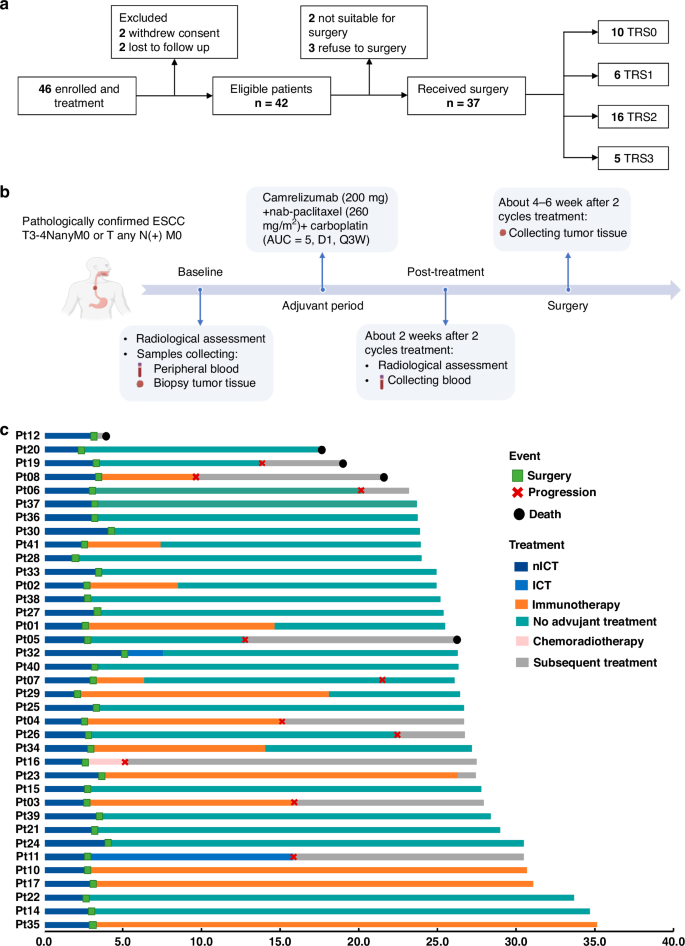
背景:食管鳞状细胞癌(ESCC)的新辅助免疫疗法正在接受深入研究。本研究评估了新辅助免疫化疗(nICT)在食管鳞癌中的疗效和免疫反应:在这项II期试验(ChiCTR2100045722)中,入组了接受nICT的局部晚期ESCC患者。主要终点是病理完全应答率(pCR)。研究人员进行了多重免疫荧光、RNA-seq和TCR-seq分析,以探索nICT的免疫反应基础:共有42名患者入组,病理完全反应率为27.0%。1年、2年的DFS和OS率分别为89.2%、64.4%和97.3%、89.2%。RNA-seq分析显示,T细胞活化是最重要的富集途径。肿瘤免疫微环境(TIME)的特点是CD4、CD8、Foxp3和PD-L1水平较高,与较好的病理消退(TRS0/1)相关。TIME分为免疫浸润型、免疫耐受型和免疫惰性型。值得注意的是,免疫浸润型和三级淋巴结构与预后改善相关。在 nICT 中,TIM-3 对疗效有负面影响,而 nICT 后 TIGIT/PD-1 表达的升高与 CD8+ T 细胞水平呈正相关。TCR-seq发现了三种TCR重排,强调了T细胞反应的特异性:结论:新辅助康瑞珠单抗加化疗对局部晚期、可切除的 ESCC 很有效,能引起深远的免疫反应,与临床结果密切相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Clinical efficacy and immune response of neoadjuvant camrelizumab plus chemotherapy in resectable locally advanced oesophageal squamous cell carcinoma: a phase 2 trial
Neoadjuvant immunotherapy is under intensive investigation for esophageal squamous cell carcinoma (ESCC). This study assesses the efficacy and immune response of neoadjuvant immunochemotherapy (nICT) in ESCC. In this phase II trial (ChiCTR2100045722), locally advanced ESCC patients receiving nICT were enrolled. The primary endpoint was the pathological complete response (pCR) rate. Multiplexed immunofluorescence, RNA-seq and TCR-seq were conducted to explore the immune response underlying nICT. Totally 42 patients were enrolled, achieving a 27.0% pCR rate. The 1-year, 2-year DFS and OS rates were 89.2%, 64.4% and 97.3%, 89.2%, respectively. RNA-seq analysis highlighted T-cell activation as the most significantly enriched pathway. The tumour immune microenvironment (TIME) was characterised by high CD4, CD8, Foxp3, and PD-L1 levels, associating with better pathological regression (TRS0/1). TIME was categorised into immune-infiltrating, immune-tolerant, and immune-desert types. Notably, the immune-infiltrating type and tertiary lymphoid structures correlated with improved outcomes. In the context of nICT, TIM-3 negatively influenced treatment efficacy, while elevated TIGIT/PD-1 expression post-nICT correlated positively with CD8+ T cell levels. TCR-seq identified three TCR rearrangements, underscoring the specificity of T-cell responses. Neoadjuvant camrelizumab plus chemotherapy is effective for locally advanced, resectable ESCC, eliciting profound immune response that closely associated with clinical outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


