降低脂肪细胞谷氨酰胺酶活性可促进能量消耗和代谢健康
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
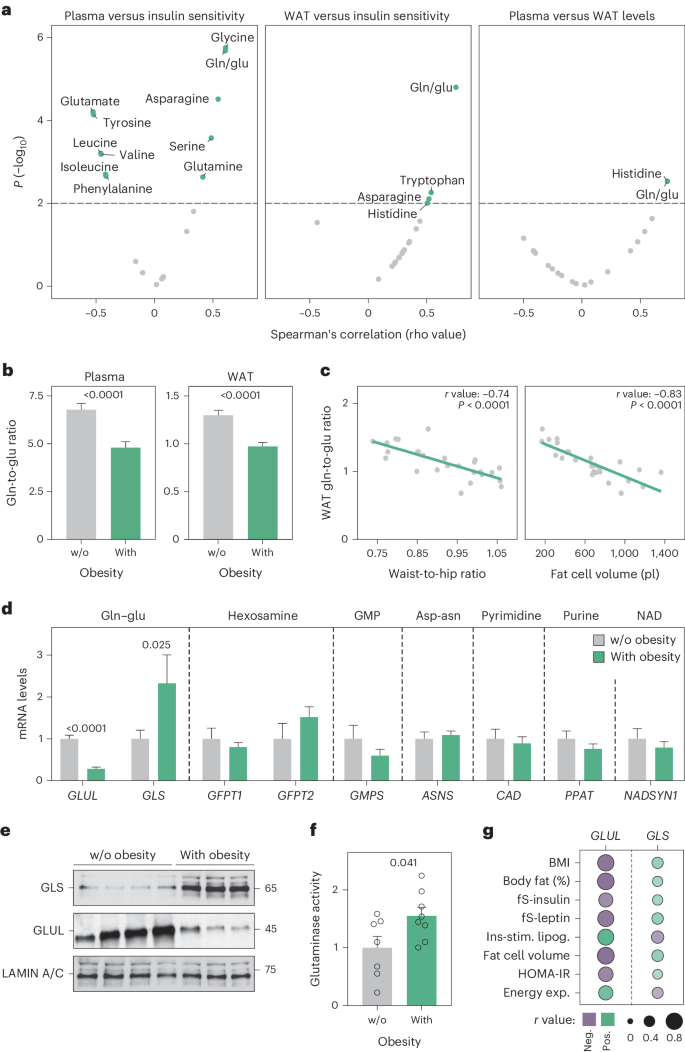
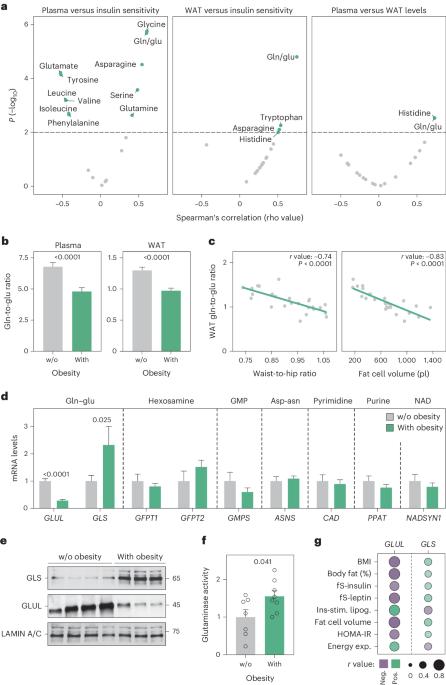
谷氨酰胺和谷氨酸通过几种酶相互转化,这种代谢循环的改变与心脏代谢特征有关。在本文中,我们发现肥胖相关的胰岛素抵抗表现为血浆和白色脂肪组织谷氨酰胺与谷氨酸比率的降低。我们将这些化学计量学变化与脂肪细胞谷氨酰胺酶和谷氨酰胺合成酶信使核糖核酸和蛋白质丰度的扰动结合起来,共同促进谷氨酰胺分解。在人类白色脂肪细胞中,谷氨酰胺酶活性的降低会通过低氧诱导因子1α丰度、乳酸水平和p38丝裂原活化蛋白激酶信号的增加促进有氧糖酵解和线粒体氧化能力。抑制雌雄小鼠体内的谷氨酰胺酶或雄性小鼠脂肪细胞中的谷氨酰胺酶基因,可激活腹股沟脂肪细胞中的致热基因程序。因此,与对照组小鼠相比,即使在高脂肪饮食条件下,基因敲除小鼠也能表现出更高的能量消耗和更好的葡萄糖耐受性。总之,我们的研究结果凸显了白脂肪细胞谷氨酰胺周转是能量消耗和代谢健康的重要决定因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reduced adipocyte glutaminase activity promotes energy expenditure and metabolic health
Glutamine and glutamate are interconverted by several enzymes and alterations in this metabolic cycle are linked to cardiometabolic traits. Herein, we show that obesity-associated insulin resistance is characterized by decreased plasma and white adipose tissue glutamine-to-glutamate ratios. We couple these stoichiometric changes to perturbed fat cell glutaminase and glutamine synthase messenger RNA and protein abundance, which together promote glutaminolysis. In human white adipocytes, reductions in glutaminase activity promote aerobic glycolysis and mitochondrial oxidative capacity via increases in hypoxia-inducible factor 1α abundance, lactate levels and p38 mitogen-activated protein kinase signalling. Systemic glutaminase inhibition in male and female mice, or genetically in adipocytes of male mice, triggers the activation of thermogenic gene programs in inguinal adipocytes. Consequently, the knockout mice display higher energy expenditure and improved glucose tolerance compared to control littermates, even under high-fat diet conditions. Altogether, our findings highlight white adipocyte glutamine turnover as an important determinant of energy expenditure and metabolic health. Lecoutre, Maqdasy and Rizo-Roca show that whole-body pharmacological inhibition or adipocyte-specific deletion of glutaminase in mice activates thermogenesis in inguinal adipocytes and promotes metabolic health. They also link decreased plasma and adipose tissue glutamine-to-glutamate ratios to insulin resistance in humans with obesity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


