Herbidomicins(草本多糖),一种由 Herbidospora 属放线菌产生的两对多酮同素异形体。
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
Herbidospora 是未被充分开发的放线菌属之一,目前仅报道了其有限的次生代谢物。在我们对探索较少的放线菌进行的持续调查中,发现 Herbidospora sp. RD 11066 的液体培养物中含有未知代谢物,而这些代谢物在我们的内部紫外数据库中没有匹配的。通过色谱分离以及随后的核磁共振和质谱结构分析,确定这些代谢物为色酮和色烯衍生物,它们分别由两种异构体的不可分割的混合物组成。前一种多酮类化合物被命名为草本呋喃酰胺 A1(1)和 A2(2),它们是芳香环上甲基取代基的位置异构体,通过两个酚羟基的缩醛交换而相互转化。后一对草本呋喃霉素 B1 (3) 和 B2 (4) 是关于烯醇醚双键的 Z/E 异构体。草本酰胺霉素 1-4 对皮肤真菌红色毛癣菌有微弱的抗真菌作用,对小鼠白血病 P388 细胞有中等程度的细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
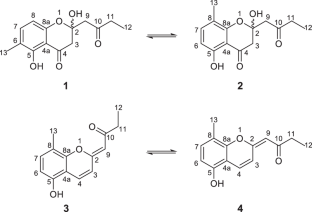
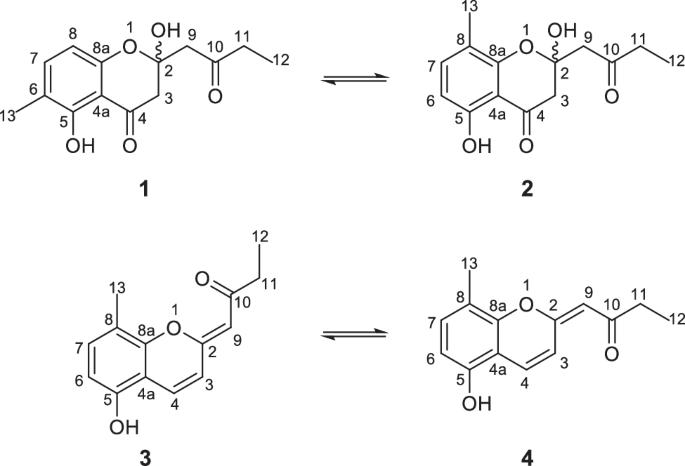
Herbidomicins, two pairs of polyketide tautomers produced by an actinomycete of the genus Herbidospora
Herbidospora is one of the underexplored actinomycete genera from which only a limited number of secondary metabolites are reported. In our continuing investigation on less explored actinomycetes, a liquid culture of Herbidospora sp. RD 11066 was found to contain unknown metabolites that had no match in our in-house UV database. Chromatographic separation and following structural analysis using NMR and MS identified these metabolites to be chromanone and chromene derivatives, which were respectively composed of an inseparable mixture of two isomeric forms. The former polyketides, designated to be herbidomicins A1 (1) and A2 (2), are positional isomers in terms of a methyl substituent on an aromatic ring that mutually interconvert by acetal exchange by two phenolic hydroxy groups. The latter pair, herbidomicins B1 (3) and B2 (4), is Z/E-isomers regarding an enol ether double bond. Herbidomicins 1–4 were weakly antifungal against a dermatophytic fungus Trichophyton rubrum and were moderately cytotoxic against murine leukemia P388 cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


