头孢唑肟是一种通过增加细胞 p27 来抑制细胞增殖的潜在药物。
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
为现有药物开发新的治疗用途对于治疗某些疾病非常重要。头孢菌素类抗生素是临床上应用最广泛的抗生素,能有效抗击细菌感染。在这里,我们发现抗菌药物头孢唑肟能通过抑制 p27 泛素化强烈上调 p27 蛋白水平。p27 蛋白是细胞周期的典型负调控因子。接下来,我们证明头孢他啶能阻碍细胞周期从G1期进入S期,从而抑制细胞增殖。此外,我们还发现头孢他啶可通过减少Skp2促进p27的表达并抑制细胞增殖,而Skp2是Skp2-Cullin-F-box(SCF)泛素连接酶的底物识别元件。此外,头孢他啶还能下调 Skp2 的转录表达。重要的是,我们证实头孢他啶能抑制体内肿瘤细胞的增殖。这些发现揭示了头孢他啶通过Skp2-p27轴介导的细胞增殖抑制作用,可为抗肿瘤治疗提供一种潜在的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
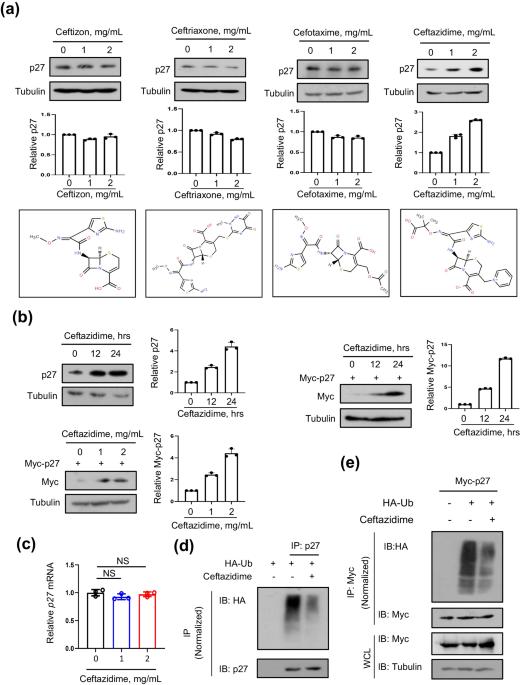
Ceftazidime is a potential drug to inhibit cell proliferation by increasing cellular p27
The development of new therapeutic uses for existing drugs is important for the treatment of some diseases. Cephalosporin antibiotics stand as the most extensively utilized antibiotics in clinical practice, effectively combating bacterial infections. Here, we found that the antimicrobial drug ceftazidime strongly upregulates p27 protein levels by inhibiting p27 ubiquitination. The p27 protein is a classic negative regulator of the cell cycle. Next, we demonstrated that ceftazidime can impede the cell cycle from G1 to S phase, thus inhibiting cell proliferation. Furthermore, we found that ceftazidime promotes p27 expression and inhibits cell proliferation by reducing Skp2, which is a substrate recognition component of the Skp2-Cullin-F-box (SCF) ubiquitin ligase. Moreover, ceftazidime downregulates transcriptional expression of Skp2. Importantly, we demonstrated that ceftazidime inhibited the proliferation of tumor cells in vivo. These findings reveal ceftazidime-mediated inhibition of cell proliferation through the Skp2-p27 axis, and could provide a potential strategy for anti-tumor therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


