氯苯基吡咯并二氮杂卓化合物的抗 MRSA 活性。
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
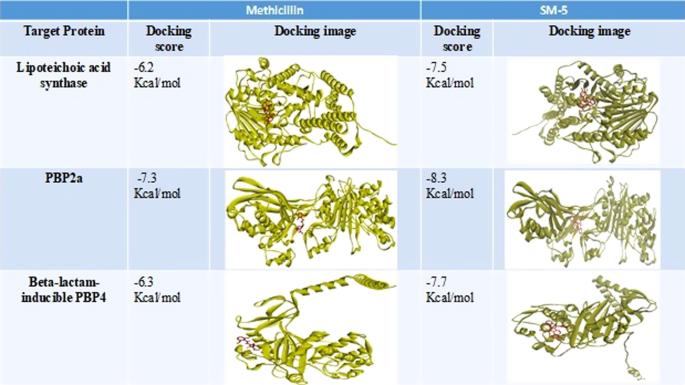
抗生素耐药性是公共卫生领域控制传染病的主要问题。MRSA(耐甲氧西林金黄色葡萄球菌)对许多抗生素(包括甲氧西林和其他β-内酰胺类药物)产生耐药性,是医疗保健领域的一个重大问题。MRSA 感染难以治疗,并增加了并发症的风险。在此,我们测试了一系列高度缩合的吡咯并[1,2-a][1,4]苯并二氮杂环衍生物。测试了化合物对革兰氏阳性菌金黄色葡萄球菌和表皮葡萄球菌以及革兰氏阴性菌大肠杆菌和绿脓杆菌的抗菌效果。与革兰氏阴性菌相比,化合物对革兰氏阳性菌的抗菌活性更强。SM-5[2-(7-(4-氯苯基)-4-甲氧基-6,7,8,13-四氢-5H-苯并[e]苯并[5,6][1,4]二氮杂卓[2,1-a]异吲哚-15-基)乙酸乙酯]衍生物被选为测试化合物中治疗指数较高的最佳化合物,对葡萄球菌菌株的 MIC 值为 7.81 微克/毫升。金黄色葡萄球菌细胞壁生物合成蛋白与 SM-5 之间的分子对接分析表明,PBP2a 的结合能最高(-8.3 Kcal mol-1),其次是β-内酰胺诱导型 PBP4(-7.7 Kcal mol-1)和脂美酸合成酶(-7.5 Kcal mol-1),与甲氧西林相当。通过 DFT 分析进行的基态能计算显示,化合物 SM-5 和 SM-6 几乎具有相等的电负性 0.11018 au,这也满足了化合物反应性的质量要求。体外生物膜抑制分析和硅学毒性分析表明,SM-5 和 SM-6 具有成为未来先导抗生素的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Anti-MRSA activity of chlorophenyl pyrrolo benzodiazepines compound
Antibiotic resistant is the major concern in public health to control the infectious diseases. MRSA (Methicillin-resistant Staphylococcus aureus) is a significant concern in healthcare settings due to its resistance to many antibiotics, including methicillin and other beta-lactams. MRSA infection difficult to treat and increases the risk of complications. Here, we have tested a series of highly condensed heterocyclic derivatives of pyrrolo[1,2-a][1,4]benzodiazepines. Compounds were tested against both, Gram-positive bacteria, Staphylococcus aureus and S. epidermidis, and Gram-negative bacteria, Escherichia coli and Pseudomonas aeruginosa, to assess the antimicrobial efficacy. Compared to Gram-negative bacteria, compounds showed much stronger antibacterial activity against Gram-positive bacteria. SM-5 [Ethyl2-(7-(4-chlorophenyl)-4-methoxy-6,7,8,13-tetrahydro-5H-benzo[e]benzo[5,6][1,4]diazepino[2,1-a]isoindol-15-yl)acetate] derivative was selected as best on the basis of higher therapeutic index among the tested compounds, showed MIC value of 7.81 µg. ml−1 against Staphylococcus strains. Molecular docking analysis between cell wall biosynthesis protein of S. aureus and SM-5 revealed that PBP2a showed the highest binding energy (−8.3 Kcal mol−1), followed by beta-lactam-inducible PBP4 (−7.7 Kcal mol−1), and lipoteichoic acid synthase (−7.5 Kcal mol−1) which is comparably higher than methicillin. Ground state energy calculations by DFT analysis revealed that compound SM-5 and SM-6, almost have equal electronegativity 0.11018 au which also satisfy the quality of the compound reactivity. Analysis of their biofilm inhibition in vitro and in silico toxicity analysis demonstrated their substantial potential to be a kind of future lead antibiotic.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


