小米离子结合能力的第一原理模型
IF 6.3
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
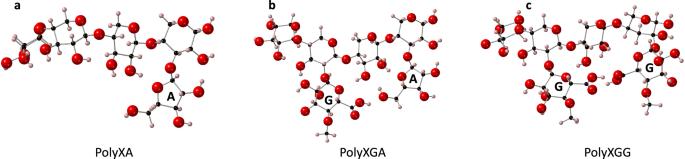
手指粟是一种在印度和非洲广泛食用的谷物,近年来因其膳食纤维(阿拉伯木聚糖)和微量矿物质含量较高以及对气候的适应能力而受到越来越多的关注。本研究旨在了解钾(K+)、钙(Ca2+)和锌(Zn2+)离子与阿拉伯木聚糖结构之间的相互作用,并确定其离子结合能力。我们构建了阿拉伯木聚糖结构拟议模型的三种变体,并进行了第一原理密度泛函理论计算,以确定阿拉伯木聚糖复合物的阳离子结合能力。在所有三种模型中,Zn2+-阿拉伯木聚糖复合物都极不稳定,热力学上也不利。然而,Ca2+ 和 K+ 离子却能形成热力学上稳定的复合物,特别是以两个葡萄糖醛酸残基作为结合袋。研究发现,葡萄糖醛酸残基在稳定阳离子-阿拉伯木聚糖复合物方面起着关键作用,立体效应比电荷密度对稳定性更为重要。我们的研究结果突出了小米纤维在离子存储能力方面最重要的结构特征,并为确证实验研究和临床试验规划提供了宝贵的初步数据,在临床试验中,可以对消化后结合离子的生物利用率进行测试。本文章由计算机程序翻译,如有差异,请以英文原文为准。
First principles modelling of the ion binding capacity of finger millet
Finger millet, a cereal grain widely consumed in India and Africa, has gained more attention in recent years due to its high dietary fibre (arabinoxylan) and trace mineral content, and its climate resilience. The aim of this study was to understand the interactions between potassium (K+), calcium (Ca2+) and zinc (Zn2+) ions and the arabinoxylan structure and determine its ion-binding capacity. Three variations of a proposed model of the arabinoxylan structure were constructed and first principles Density Functional Theory calculations were carried out to determine the cation-binding capacity of the arabinoxylan complexes. Zn2+-arabinoxylan complexes were highly unstable and thermodynamically unfavourable in all three models. Ca2+ and K+ ions, however, form thermodynamically stable complexes, particularly involving two glucuronic acid residues as a binding pocket. Glucuronic acid residues are found to play a key role in stabilising the cation-arabinoxylan complex, and steric effects are more important to the stability than charge density. Our results highlight the most important structural features of the millet fibre regarding ion-storage capacity, and provide valuable preliminary data for confirmatory experimental studies and for the planning of clinical trials where the bioavailability of bound ions following digestion may be tested.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Science of Food
FOOD SCIENCE & TECHNOLOGY-
CiteScore
7.50
自引率
1.60%
发文量
53
期刊介绍:
npj Science of Food is an online-only and open access journal publishes high-quality, high-impact papers related to food safety, security, integrated production, processing and packaging, the changes and interactions of food components, and the influence on health and wellness properties of food. The journal will support fundamental studies that advance the science of food beyond the classic focus on processing, thereby addressing basic inquiries around food from the public and industry. It will also support research that might result in innovation of technologies and products that are public-friendly while promoting the United Nations sustainable development goals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


