在活跃的人类启动子上,转录方向性由 Integrator 许可
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
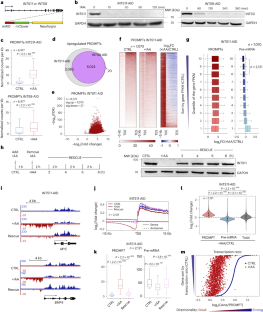
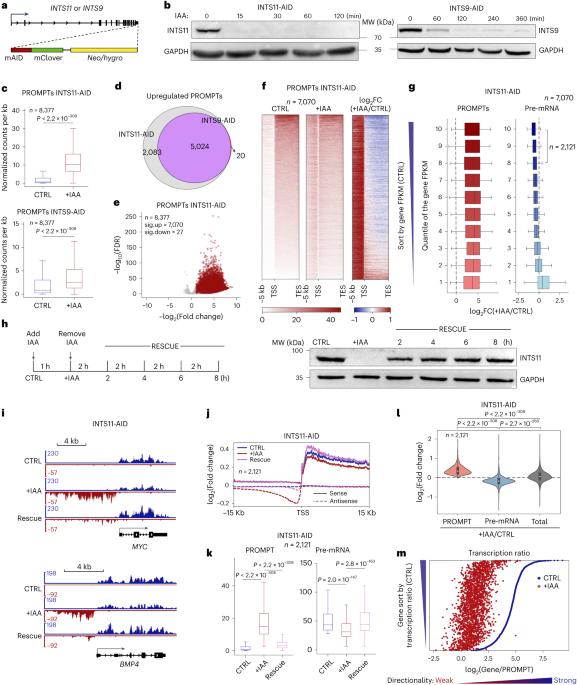
真核生物转录的一个普遍特征是启动子招募 RNA 聚合酶 II(RNAPII)产生前体 mRNA(pre-mRNA)和短的不稳定启动子上游转录本(PROMPT),朝相反的方向转录。然而,转录机制如何选择正确的方向来产生前 mRNA 在很大程度上是未知的。在这里,我们通过多种急性辅助素诱导的降解系统表明,RNAPII结合蛋白复合物Integrator的快速耗竭会导致PROMPT在整个基因组中的大量积累。有趣的是,PROMPTs 的积累是通过减少活跃转录基因中的前 mRNA 转录本来补偿的。同样,Integrator 的耗竭改变了聚合酶在有义和反义方向之间的分布,这表现为 RNAPII 羧基末端结构域 Tyr1 在 PROMPT 区域的磷酸化水平增加,而在转录起始位点的 Ser2 磷酸化水平降低。从机理上讲,Integrator 的内切酶活性对抑制 PROMPT 的产生至关重要。此外,我们的数据表明,新生转录本上存在的 U1 结合位点可以抵消 Integrator 的裂解活性。在这一过程中,由于大多数 PROMPT 上缺乏强有力的 U1 信号,Integrator 可以抑制反义转录,并使转录平衡向有义方向倾斜。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Transcription directionality is licensed by Integrator at active human promoters
A universal characteristic of eukaryotic transcription is that the promoter recruits RNA polymerase II (RNAPII) to produce both precursor mRNAs (pre-mRNAs) and short unstable promoter upstream transcripts (PROMPTs) toward the opposite direction. However, how the transcription machinery selects the correct direction to produce pre-mRNAs is largely unknown. Here, through multiple acute auxin-inducible degradation systems, we show that rapid depletion of an RNAPII-binding protein complex, Integrator, results in robust PROMPT accumulation throughout the genome. Interestingly, the accumulation of PROMPTs is compensated by the reduction of pre-mRNA transcripts in actively transcribed genes. Consistently, Integrator depletion alters the distribution of polymerase between the sense and antisense directions, which is marked by increased RNAPII-carboxy-terminal domain Tyr1 phosphorylation at PROMPT regions and a reduced Ser2 phosphorylation level at transcription start sites. Mechanistically, the endonuclease activity of Integrator is critical to suppress PROMPT production. Furthermore, our data indicate that the presence of U1 binding sites on nascent transcripts could counteract the cleavage activity of Integrator. In this process, the absence of robust U1 signal at most PROMPTs allows Integrator to suppress the antisense transcription and shift the transcriptional balance in favor of the sense direction. The authors investigate how the transcription machinery selects the correct direction to produce coding transcripts. Their results propose a universal mechanism by which Integrator licenses bidirectional transcription to determine the direction of eukaryotic pre-mRNA transcription.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


