预测孔隙约束下氢在水中的溶解度将增加十倍
IF 15
2区 环境科学与生态学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
地质构造中的地下氢储存作为全球能源转型的一种潜在解决方案,已受到越来越多的关注。氢在地下封闭条件下的溶解度变化是安全和效率的关键挑战,但目前有关氢在粘土纳米封闭条件下的溶解度的知识还很少。在此,我们利用分子动力学模拟研究了在高岭石的约束下,氢在实际储存条件下的水溶性。我们发现,对于疏水和亲水系统,在纳米尺度约束下的溶解度都比在体液中的溶解度提高了十倍。我们讨论了这种超溶解性的驱动机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
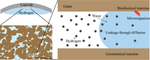
Predicted tenfold increase of hydrogen solubility in water under pore confinement
Underground hydrogen storage in geological formations has gained interest as a potential solution for the global energy transition. The change of hydrogen solubility in underground confinement is a key challenge for safety and efficiency, yet there is few knowledge on hydrogen solubility under nanoconfinement in clays. Here we used molecular dynamic simulations to study hydrogen solubility in water at realistic storage conditions under the confinement of kaolinite. We find a solubility enhancement of tenfold under nanoscale confinement compared with that in the bulk for both hydrophobic and hydrophilic systems. Mechanisms driving this oversolubility are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Chemistry Letters
环境科学-工程:环境
CiteScore
32.00
自引率
7.00%
发文量
175
审稿时长
2 months
期刊介绍:
Environmental Chemistry Letters explores the intersections of geology, chemistry, physics, and biology. Published articles are of paramount importance to the examination of both natural and engineered environments. The journal features original and review articles of exceptional significance, encompassing topics such as the characterization of natural and impacted environments, the behavior, prevention, treatment, and control of mineral, organic, and radioactive pollutants. It also delves into interfacial studies involving diverse media like soil, sediment, water, air, organisms, and food. Additionally, the journal covers green chemistry, environmentally friendly synthetic pathways, alternative fuels, ecotoxicology, risk assessment, environmental processes and modeling, environmental technologies, remediation and control, and environmental analytical chemistry using biomolecular tools and tracers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


