Thâmara Carollyne de Luna Rocha , Maria Joanellys dos Santos Lima , Jéfferson Luan Nunes do Nascimento , Jamerson Ferreira de Oliveira , Emerson de Oliveira Silva , Victor Hugo Barbosa dos Santos , André de Lima Aires , Victor de Albuquerque Wanderley Sales , Talita Atanazio Rosa , Pedro José Rolim Neto , Mônica Camelo Pessôa de Azevedo Albuquerque , Maria do Carmo Alves de Lima , Rosali Maria Ferreira da Silva
求助PDF
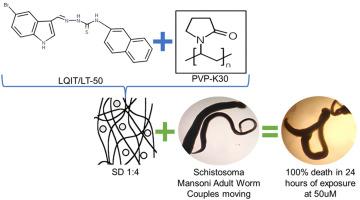
{"title":"开发和评估基于 2-(-5-溴-1-H-吲哚-3-基亚甲基)-N-(萘-1-基肼-carbothiamide)的固体分散体的体外杀血吸虫活性","authors":"Thâmara Carollyne de Luna Rocha , Maria Joanellys dos Santos Lima , Jéfferson Luan Nunes do Nascimento , Jamerson Ferreira de Oliveira , Emerson de Oliveira Silva , Victor Hugo Barbosa dos Santos , André de Lima Aires , Victor de Albuquerque Wanderley Sales , Talita Atanazio Rosa , Pedro José Rolim Neto , Mônica Camelo Pessôa de Azevedo Albuquerque , Maria do Carmo Alves de Lima , Rosali Maria Ferreira da Silva","doi":"10.1016/j.exppara.2023.108626","DOIUrl":null,"url":null,"abstract":"<div><p><span><span><span>Among all the neglected diseases, schistosomiasis is considered the second most important parasitic infection after malaria. Praziquantel is the most widely used drug for this disease, but its exclusive use may result in the development of drug-resistant schistosomiasis. To increase the control of the disease, new drugs have been developed as alternative treatments, among them 2-(-5-bromo-1-h-indole-3-yl-methylene)-N-(naphthalene-1-ylhydrazine-carbothiamide (LQIT/LT-50), which showed promising schistosomicidal activity in </span>nonclinical studies. However, LQIT/LT-50 presents low solubility in water, resulting in reduced bioavailability. To overcome this solubility problem, the present study aimed to develop LQIT/LT-50 solid dispersions for the treatment of schistosomiasis. Solid dispersions were prepared through the solvent method using Soluplus©, polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP K-30) as hydrophilic carriers. The formulations with the best results in the compatibility tests, aqueous solubility and preliminary stability studies have undergone solubility tests and physicochemical characterizations by Fourier-transform infrared spectroscopy (FTIR), x-ray diffractometry (XRD), exploratory differential calorimetry (DSC), thermogravimetry (TG) and </span>Raman spectroscopy. Finally, the schistosomicidal activity was evaluated </span><em>in vitro</em>. The phycochemical analyzes showed that when using PVP K-30, there was an interaction between the PVP K-30 and LQIT/LT-50, proving the successful development of the solid dispersion. Furthermore, an increase in the solubility of the new system was observed (LQIT/LT-50:PVP K-30) in addition to the improvement in the <em>in vitro</em> shistosomidal activity at 1:4 (w/w) molar ratio (i.e., 20% drug loading) when compared to LQIT/LT-50 alone. The development of the LQIT/LT-50:PVP K-30 1:4 solid dispersion is encouraging for the future development of new pharmaceutical solid formulations, aiming the schistosomicidal treatment.</p></div>","PeriodicalId":12117,"journal":{"name":"Experimental parasitology","volume":null,"pages":null},"PeriodicalIF":1.4000,"publicationDate":"2023-11-14","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Development and evaluation of the in vitro schistosomicidal activity of solid dispersions based on 2-(-5-bromo-1-H-indole-3-yl-methylene)-N-(naphthalene-1-ylhydrazine-carbothiamide\",\"authors\":\"Thâmara Carollyne de Luna Rocha , Maria Joanellys dos Santos Lima , Jéfferson Luan Nunes do Nascimento , Jamerson Ferreira de Oliveira , Emerson de Oliveira Silva , Victor Hugo Barbosa dos Santos , André de Lima Aires , Victor de Albuquerque Wanderley Sales , Talita Atanazio Rosa , Pedro José Rolim Neto , Mônica Camelo Pessôa de Azevedo Albuquerque , Maria do Carmo Alves de Lima , Rosali Maria Ferreira da Silva\",\"doi\":\"10.1016/j.exppara.2023.108626\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<div><p><span><span><span>Among all the neglected diseases, schistosomiasis is considered the second most important parasitic infection after malaria. Praziquantel is the most widely used drug for this disease, but its exclusive use may result in the development of drug-resistant schistosomiasis. To increase the control of the disease, new drugs have been developed as alternative treatments, among them 2-(-5-bromo-1-h-indole-3-yl-methylene)-N-(naphthalene-1-ylhydrazine-carbothiamide (LQIT/LT-50), which showed promising schistosomicidal activity in </span>nonclinical studies. However, LQIT/LT-50 presents low solubility in water, resulting in reduced bioavailability. To overcome this solubility problem, the present study aimed to develop LQIT/LT-50 solid dispersions for the treatment of schistosomiasis. Solid dispersions were prepared through the solvent method using Soluplus©, polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP K-30) as hydrophilic carriers. The formulations with the best results in the compatibility tests, aqueous solubility and preliminary stability studies have undergone solubility tests and physicochemical characterizations by Fourier-transform infrared spectroscopy (FTIR), x-ray diffractometry (XRD), exploratory differential calorimetry (DSC), thermogravimetry (TG) and </span>Raman spectroscopy. Finally, the schistosomicidal activity was evaluated </span><em>in vitro</em>. The phycochemical analyzes showed that when using PVP K-30, there was an interaction between the PVP K-30 and LQIT/LT-50, proving the successful development of the solid dispersion. Furthermore, an increase in the solubility of the new system was observed (LQIT/LT-50:PVP K-30) in addition to the improvement in the <em>in vitro</em> shistosomidal activity at 1:4 (w/w) molar ratio (i.e., 20% drug loading) when compared to LQIT/LT-50 alone. The development of the LQIT/LT-50:PVP K-30 1:4 solid dispersion is encouraging for the future development of new pharmaceutical solid formulations, aiming the schistosomicidal treatment.</p></div>\",\"PeriodicalId\":12117,\"journal\":{\"name\":\"Experimental parasitology\",\"volume\":null,\"pages\":null},\"PeriodicalIF\":1.4000,\"publicationDate\":\"2023-11-14\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Experimental parasitology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://www.sciencedirect.com/science/article/pii/S0014489423001674\",\"RegionNum\":4,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q3\",\"JCRName\":\"PARASITOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Experimental parasitology","FirstCategoryId":"3","ListUrlMain":"https://www.sciencedirect.com/science/article/pii/S0014489423001674","RegionNum":4,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"PARASITOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


