硝基取代给受体环丙烷一锅法合成β-氨基-γ-酮丙二酸酯
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
建立了从硝基取代的给受体环丙烷和四种不同的n -化合物中获得β-氨基-γ-酮丙二酸酯的顺序一锅法。该反应通过DMSO的Kornblum型开环氧化和随后的aza-Michael加成,由硝基环丙烷原位生成芳基甲基己酸酯。为了证明所得产物的合成效用,将其中一种转化为吡嗪酮衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
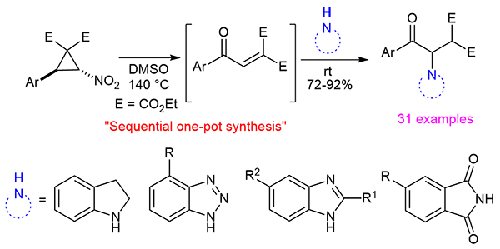
Sequential One-Pot Synthesis of β-Amino-γ-keto-malonates from Nitro-Substituted Donor-Acceptor Cyclopropanes
A sequential one-pot procedure has been developed for the access of β-Amino-γ-keto-malonates from nitro-substituted donor-acceptor cyclopropanes and four different N-compounds. The reaction proceeds through in situ generation of aroylmethylidenemalonates from the nitro cyclopropanes via Kornblum type ring-opening oxidation using DMSO and subsequent aza-Michael addition with the N-compounds. To prove the synthetic utility of the resulting products, one of them was transformed into a pyridazinone derivative.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


