7A型肺炎链球菌荚膜多糖六糖重复单元的快速合成
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
摘要采用[3+2+1]阻滞合成策略,以较好的收率合成了肺炎链球菌7A型荚膜多糖对应的六糖重复单元。该合成策略涉及许多具有挑战性的立体选择性糖基化步骤,包括β-选择性糖基化l-鼠李糖基硫甙供体,α-选择性糖基化2-叠氮-2-脱氧-d-葡萄糖吡喃糖基硫苷供体和d-半乳糖吡喃糖基供体,以及β-半乳糖胺苷段和α-鼠李糖基苷段的形成。适当功能化的硫代糖苷被用作糖基供体,n -碘琥珀酰亚胺(NIS)和三甲基硅基三氟甲烷磺酸盐(TMSOTf -)的组合被用作糖基化启动子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
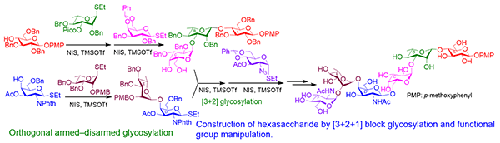
Expeditious Synthesis of the Hexasaccharide Repeating Unit of the Capsular Polysaccharide of Streptococcus pneumoniae Type 7A
Abstract The hexasaccharide repeating unit corresponding to the capsular polysaccharide of Streptococcus pneumoniae type 7A has been synthesized in good yield using [3+2+1] block synthetic strategy. The synthetic strategy involved a number of challenging stereoselective glycosylation steps, which include β-selective glycosylation of l-rhamnosyl thioglycoside donor, α-selective glycosylations of 2-azido-2-deoxy-d-glucopyranosyl thioglycoside donor and d-galactopyranosyl donor together with the formation of β-glycoside of d-galactosamine moiety and α-glycoside of l-rhamnosyl moiety. Suitably functionalized thioglycosides have been used as glycosyl donors and a combination of N-iodosuccinimide (NIS) and trimethylsilyl trifluoromethanesulfonate (TMSOTf) has been used as glycosylation promoter.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


