瑞香花素G和H,抗hiv大环3,4-邻瑞香烷正酯。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从长柄达芙妮中分离到具有特殊大环3,4-邻达芙妮烷正构酯结构的水仙花苷G(1)和H(2)。通过物理化学和光谱分析,结合甲基酯化、手性各向异性衍生化反应和仿生转化等合成方法确定了它们的结构。对化合物1和2及其甲酯1a和2a的抗hiv活性进行了评价,其中1a和2a的IC50值分别为1.08和1.17 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
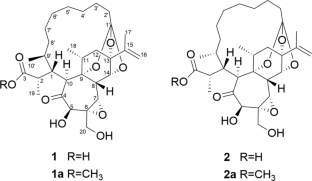
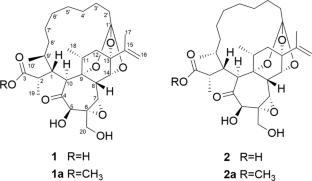
Daphnepedunins G and H, anti-HIV macrocyclic 3,4-seco-daphnane orthoesters from Daphne pedunculata
Daphnepedunins G (1) and H (2) with unusual macrocyclic 3,4-seco-daphnane orthoester structure were isolated from Daphne pedunculata. Their structures were determined by physicochemical and spectroscopic analyses combined with synthetic methods, including methyl esterification, derivatization reaction using a chiral anisotropic agent, and biomimetic conversion. Compounds 1 and 2 along with their methyl esters 1a and 2a were evaluated for anti-HIV activity, among which 1a and 2a exhibited potent activity with IC50 values of 1.08 and 1.17 μM, respectively.
Graphical abstract
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
3.00%
发文量
79
审稿时长
1.7 months
期刊介绍:
The Journal of Natural Medicines is an international journal publishing original research in naturally occurring medicines and their related foods and cosmetics. It covers:
-chemistry of natural products
-biochemistry of medicinal plants
-pharmacology of natural products and herbs, including Kampo formulas and traditional herbs
-botanical anatomy
-cultivation of medicinal plants.
The journal accepts Original Papers, Notes, Rapid Communications and Natural Resource Letters. Reviews and Mini-Reviews are generally invited.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


