CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 566
Abstract
CMTM6 maintains PD-L1 at the plasma membrane by inhibiting its lysosome-mediated degradation and promoting its recycling. Understanding the molecular regulation of programmed death-1 ligand 1 (PD-L1) expression could help to explain the success of certain anti-tumour therapies that disrupt PD-L1-mediated tumour tolerance. Mark Dawson and colleagues identify a novel regulator of PD-L1 expression, CMTM6, through a genome-wide CRISPR–Cas9 screen. CMTM6 functions to maintain PD-L1 at the plasma membrane by inhibiting its lysosome-mediated degradation and promoting its recycling. Elsewhere in this issue, Ton Schumacher and colleagues describe a haploid genetic screen to identify molecules and pathways that influence the cell surface expression of PD-L1. They also identify chemokine-like factors CMTM6 and CMTM4 as cell endogenous regulators of PD-L1 stability, and suggest that this axis could be targeted therapeutically to improve cancer immunotherapy. Cancer cells exploit the expression of the programmed death-1 (PD-1) ligand 1 (PD-L1) to subvert T-cell-mediated immunosurveillance1,2. The success of therapies that disrupt PD-L1-mediated tumour tolerance has highlighted the need to understand the molecular regulation of PD-L1 expression1. Here we identify the uncharacterized protein CMTM6 as a critical regulator of PD-L1 in a broad range of cancer cells, by using a genome-wide CRISPR–Cas9 screen. CMTM6 is a ubiquitously expressed protein that binds PD-L1 and maintains its cell surface expression. CMTM6 is not required for PD-L1 maturation but co-localizes with PD-L1 at the plasma membrane and in recycling endosomes, where it prevents PD-L1 from being targeted for lysosome-mediated degradation. Using a quantitative approach to profile the entire plasma membrane proteome, we find that CMTM6 displays specificity for PD-L1. Notably, CMTM6 depletion decreases PD-L1 without compromising cell surface expression of MHC class I. CMTM6 depletion, via the reduction of PD-L1, significantly alleviates the suppression of tumour-specific T cell activity in vitro and in vivo. These findings provide insights into the biology of PD-L1 regulation, identify a previously unrecognized master regulator of this critical immune checkpoint and highlight a potential therapeutic target to overcome immune evasion by tumour cells.
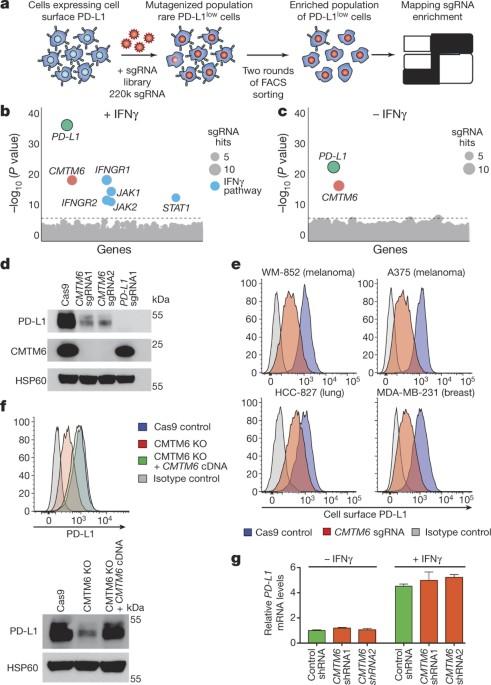
CMTM6 可维持 PD-L1 的表达并调节抗肿瘤免疫力
CMTM6通过抑制溶酶体介导的PD-L1降解并促进其循环,将其维持在质膜上。了解程序性死亡-1配体1(PD-L1)表达的分子调控有助于解释某些破坏PD-L1介导的肿瘤耐受性的抗肿瘤疗法取得成功的原因。Mark Dawson及其同事通过全基因组CRISPR-Cas9筛选发现了一种新型PD-L1表达调控因子CMTM6。CMTM6 的功能是通过抑制溶酶体介导的降解和促进其循环,将 PD-L1 维持在质膜上。在本期其他文章中,Ton Schumacher 及其同事介绍了一种单倍体基因筛选方法,以确定影响 PD-L1 细胞表面表达的分子和途径。他们还发现趋化因子样因子CMTM6和CMTM4是PD-L1稳定性的细胞内源调节因子,并建议可以针对这一轴心进行治疗,以改善癌症免疫疗法。癌细胞利用程序性死亡-1(PD-1)配体1(PD-L1)的表达来颠覆T细胞介导的免疫监视1,2。破坏 PD-L1 介导的肿瘤耐受性的疗法取得了成功,这凸显了了解 PD-L1 表达的分子调控的必要性1。在这里,我们通过全基因组 CRISPR-Cas9 筛选,发现了未表征的蛋白 CMTM6 是 PD-L1 在多种癌细胞中的关键调控因子。CMTM6是一种泛在表达的蛋白质,能结合PD-L1并维持其细胞表面表达。CMTM6不是PD-L1成熟所必需的,但它与PD-L1共定位在质膜和循环内体中,防止PD-L1成为溶酶体介导的降解靶标。通过定量分析整个质膜蛋白质组,我们发现 CMTM6 对 PD-L1 具有特异性。值得注意的是,CMTM6 的消耗会减少 PD-L1 而不影响细胞表面 MHC I 类的表达。这些发现深入揭示了 PD-L1 调控的生物学原理,确定了这一关键免疫检查点的一个先前未被发现的主调控因子,并突出强调了克服肿瘤细胞免疫逃避的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


