Studies on the Mechanism of Styrene Elimination from Sulfilimines: Hammett and Kinetic Isotope Effect Analysis and Computation
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
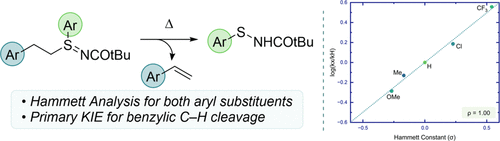
N-Acylsulfenamides can be efficiently prepared by a one-pot synthetic sequence via S-functionalization of N-acyl S-phenethyl sulfenamides, utilizing diverse (hetero)aryl and alkenyl iodide and boronic acid inputs to give sulfilimines that then undergo concerted elimination of styrene. To probe the mechanism of styrene elimination from sulfilimines, a Hammett analysis was performed with substituents placed on both the S-aryl and S-phenethyl groups. Positive Hammett ρ values established a modest negative charge build up at both positions, with greater charge buildup at the phenethyl site. A primary isotope effect of 5.4 at the benzyl site was also determined through the synthesis of a deuterated sulfilimine and is consistent with significant C–H bond cleavage at the benzylic carbon in the transition state. Computation provides strong support for the proposed concerted elimination mechanism.
亚砜亚胺中苯乙烯消去机理的研究:Hammett及动力学同位素效应分析与计算
n -酰基- s -苯基亚胺可以通过n -酰基- s -苯基亚胺的s -功能化,利用不同的(杂)芳基和烯基碘化物和硼酸输入得到亚胺,然后经过协调消除苯乙烯,通过一锅合成序列有效地制备n -酰基亚胺。为了探究苯乙烯从亚砜亚胺中消除的机理,在s -芳基和s -苯基上放置取代基进行了Hammett分析。正的哈米特ρ值在两个位置都建立了适度的负电荷,在苯乙基位置有更大的电荷积累。通过合成氘化亚胺,还确定了苯基上的主要同位素效应为5.4,这与过渡态苯基碳上的C-H键裂解一致。计算结果为提出的协同消除机制提供了强有力的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


