Exploring a New Ruthenium(II) Complex with High DNA Binding Ability as a Novel Efficient Luminescent Intercalation Agent to Construct a Label-Free ECL/PL Dual-Mode Biosensor
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
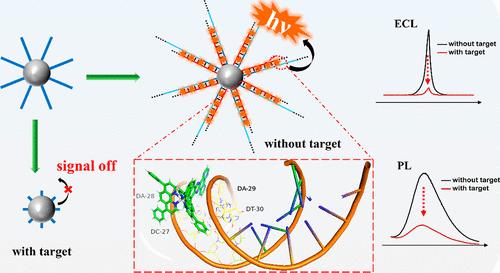
Ruthenium(II) complexes with special ligands have been widely recognized in numerous fields and attributed to their outstanding DNA binding capacity. Hybridization chain reaction (HCR), as an enzyme-free amplification technique, forms long double-stranded DNA (dsDNA) structures, which provides an intercalation platform for these complexes and obtains an effective enhancement of luminescent signals to a significant extent and enhances the sensitivity of detection. Hence, Ru(dip)2(tpphz) [dip = 4,7-diphenyl-1,10-phenanthroline, tpphz = tetrapyrido[3,2-a:2′,3′-c:3″,2″-h:2‴,3‴-j]phenazine] confirmed to possess high DNA binding capacity via UV–vis absorption spectroscopy and AutoDock theoretical simulation calculations was synthesized as a luminescence probe. As a proof of concept, the label-free ECL/PL dual-mode biosensor was further constructed. In this design, magnetic silica spheres with trigger DNA were amplified by HCR with hairpin DNA, forming large amounts of dsDNA on the surface, and Ru(dip)2(tpphz) was incorporated to generate robust signals. Trigger DNA was cleaved owing to the activation of Cas12a cleavage ability in the presence of the target, HCR amplification disappeared, and the signals reduced. The biosensor exhibited high selectivity, and the LOD was as low as 69 fM (S/N = 3). The results proved that Ru(dip)2(tpphz) has excellent DNA binding ability and ECL and PL dual properties, which has huge potential to establish label-free dual-mode biosensors and simultaneously offers a tremendous prospect in the fields of anticancer, gene therapy, and molecular probes beyond label-free biosensors in the future.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


