High-Efficiency Photooxidation of Methane to the C1 Product
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
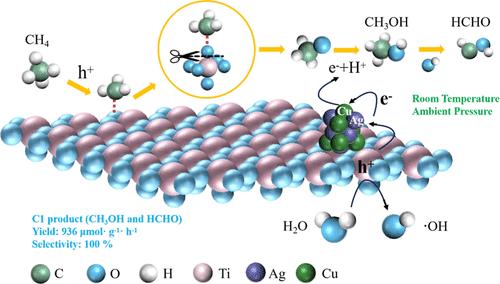
The efficient conversion of methane (CH4) to high-value-added chemicals using a photocatalyst at room temperature and pressure faces great challenges compared to harsh reaction conditions. However, achieving this efficient conversion would yield substantial cost advantages and hold immense potential for development. Here, we demonstrate the enhanced photocatalytic conversion efficiency of CH4 at room temperature and pressure conditions without requiring any oxidant through the construction of a bimetal Ag–Cu-loaded brookite TiO2 photocatalyst. The C1 products were ultimately obtained with 100% selectivity and a yield of 936 μmol·g–1·h–1. The performance exceeds that of similar research by tens of times. The high selectivity of this system is attributed to the optimal number of ·OH, which strikes a balance between excess and deficiency. Ag effectively enhances electron transport in the photocatalytic reaction process on a dual active site photocatalyst, while Cu significantly improves the selectivity of the C1 products. In this system, the hydroxyl radical (·OH) activates CH4 to generate the methyl radical (·CH3), which then binds with the lattice oxygen of TiO2, breaking the Ti–O bond and resulting in the formation of *OCH3. The *OCH3 undergoes further conversion to CH3OH, which is subsequently oxidized to HCHO by ·OH. This work presents a cost-effective and highly efficient approach for directly oxidizing CH4 into valuable chemicals, ensuring superior selectivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


