Coassembly of Enzyme-Responsive Cholate-Conjugated Facially Amphiphilic Polymers
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
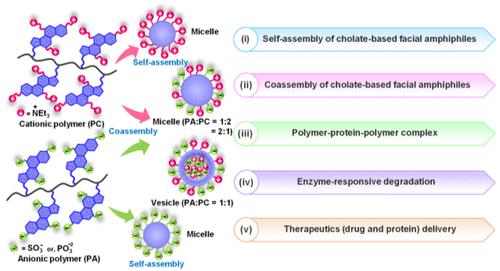
Inspired by the unique self-assembly of bile acid-based facial amphiphiles (FAs), a family of facially amphiphilic cholic acid-derived homopolymers and their coassembled nanoaggregates have been fabricated. The cholate-pendant cationic PC (quaternary amine-based), anionic PA1 (sulfate-based), and PA2 (phosphate-based) polymers individually formed spherical nanoaggregates due to the required hydrophobic/hydrophilic balance. However, the coassembled aggregates of oppositely charged pairs, PC with PA1 or PA2, generated arrays of different nanoaggregates depending on the mixing ratio. Additionally, the polymer–protein–polymer coassembly among two oppositely charged facially amphiphilic polymers and a positively/negatively charged therapeutic protein (lysozyme or insulin) was thoroughly investigated by the UV–vis turbidimetry assay, transmission electron microscopy, and dynamic light scattering. The presence of ester, sulfate, and phosphate groups in PC, PA1, and PA2 makes them susceptible to degradation by esterase, sulfatase, and phosphatase enzymes, respectively. Thus, enzyme-triggered in vitro release of two different therapeutics, small-molecule drugs (doxorubicin, DOX) and biomacromolecules (insulin and lysozyme), has been showcased from the self-assembled and coassembled nanoaggregates in physiological conditions. Overall, the present work successfully demonstrated the development of coassembled nanoaggregates using the two oppositely charged cholate-based FAs, with a promising potential for engineering next-generation enzyme-responsive therapeutic delivery vehicles.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


