Remarkable Increase in the Rate of Trans–Cis Photoisomerization of Os(II)-Terpyridine Complexes via Oxidation and Reduction
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
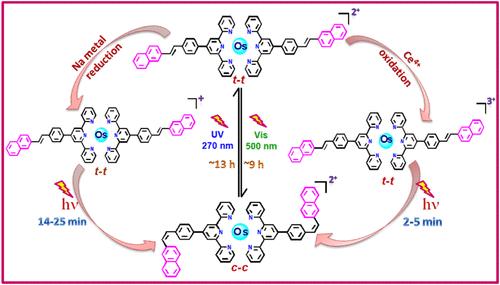
Luminescent homoleptic Os(II)-terpyridine complexes comprising stilbene-appended naphthalene, anthracene, and pyrene motifs are designed in this work, and their photophysical, electrochemical, and photoisomerization behaviors are extensively investigated. All complexes exhibit intense spin-allowed singlet metal-to-ligand charge transfer (1MLCT) bands in the visible (496–500 nm) and weaker spin-forbidden singlet-to-triplet 3MLCT transitions in the 600–700 nm range. They display moderate emission at room temperature with lifetimes in the range of 84.5–112.5 ns. Electrochemical studies reveal a reversible Os2+/Os3+ oxidation couple within 0.93–0.96 V, alongside multiple reversible or quasi-reversible reduction peaks associated with terpyridine units in between −1.10 and −1.85 V. The stilbene motifs facilitate reversible trans–cis photoisomerization under alternative treatment with visible and UV light, enabling the complexes to function as photomolecular switches in the near-infrared domain. Interestingly, a remarkable increase in the rate of photoisomerization has been achieved via oxidation as well as reduction of the complexes, which, in turn, induces multistep switching involving reversible oxidation–reduction and trans–cis isomerization. Computational investigations are also conducted on all three conformations {trans–trans (t–t), trans–cis (t–c), and cis–cis (c–c)} of the complexes to gain insight into their electronic structures and for accurate assignment of their absorption and emission spectral bands.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


